Scientists have uncovered a new biomedical technique that could increase blood flow to alleviate problems associated with cardiovascular disease, diabetes and many surgical procedures. They found that blocking the action of a blood-clot-associated protein turns up the effect of a biologically produced gas that can open blood vessels and increase blood flow.
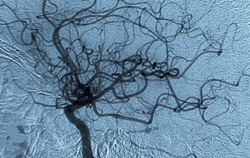
The researchers showed that the protein, thrombospondin-1, inhibits the effect of the gas, nitric oxide (NO), once named Molecule of the Year by Science magazine because of its many positive effects on cardiovascular health. Decreasing thrombospondin-1’s suppression enhanced the effects of NO.
The researchers, including William A. Frazier, Ph.D., at Washington University School of Medicine in St. Louis, believe their findings ultimately could find application for wide-ranging medical problems such as burn treatments, organ transplants, heart attacks, strokes and peripheral vascular disease, a severe complication of diabetes.
Thrombospondin-1 is named for the Latin term thrombos, meaning blood clot, and is shed by platelets in the bloodstream when they are activated to form a clot. It is also synthesized at low levels by every cell in the vascular system. Interestingly, the protein was actually discovered decades ago at the School of Medicine in the laboratory of Philip Majerus, M.D., professor of medicine and of biochemistry and molecular biophysics, but in spite of its wide distribution in the body, its function has been difficult to identify.
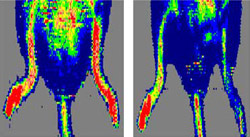
Now Frazier and colleagues including David D. Roberts, Ph.D., and Jeff S. Isenberg, M.D., at the National Cancer Institute (NCI) have uncovered a connection between thrombospondin-1 and NO, a molecule found throughout the circulatory system and the subject of innumerable scientific investigations.
NO acts very quickly to expand or dilate blood vessels to provide more oxygen to tissues. NO is also important in angiogenesis, the longer-term response to oxygen deprivation that results in new blood vessels reaching the affected area.
“NO is a master regulator of vascular health. Abnormalities in its production are the cause of or a contributor to nearly every cardiovascular disease,” says Frazier, professor of biochemistry and molecular biophysics and of cell biology and physiology and a researcher with the Siteman Cancer Center at Washington University and Barnes-Jewish Hospital. “Our colleagues at NCI showed that if you knock out thrombospondin-1, you can greatly increase the effect of NO, not only to stimulate angiogenesis, but also to enhance blood circulation and tissue recovery in several mouse models of tissue ischemia.”
Frazier and his research group at Washington University identified a mediator of thrombospondin-1’s effect on NO. They found that a protein called CD47, present on the surface of all cells in the blood vessel system, is the receptor that binds thrombospondin-1 and allows it to inhibit NO signaling. In addition, they found that a small fragment, a peptide of only 10 amino acids out of the almost 1,200 amino acids comprising thrombospondin-1, is all that is needed to trigger CD47. Now researchers believe they can control this mechanism and increase NO signaling to relax blood vessels to increase blood flow and to encourage the growth of new capillary vessels.

“Normally, thrombospondin-1 — via CD47 — limits the effect of NO on blood vessel dilation and angiogenesis,” Frazier says. “So if you treated a patient with an NO donor compound, the patient’s own thrombospondin/CD47 system would put a lid on how much benefit could be achieved. But when CD47 is inhibited or eliminated by knocking out the gene in laboratory mice, there is a substantial improvement in tissue oxygenation.”
This is due to blood vessel dilation in the short term and angiogenesis in the longer term, according to Frazier.
“The dramatic effects on tissue viability seen in the experiments by our NCI collaborators may be telling us that interfering with the thrombospondin-1/CD47 signaling axis could benefit heart and stroke patients and patients with diabetes, as well as improving the outcome of surgical procedures and burn treatments,” Frazier says.
Frazier and his collaborators indicate that the peptide of thrombospondin-1 that interacts with CD47 and other reagents that target CD47 will be the starting point for developing new drugs that could improve the outcome of medical procedures in which tissue oxygenation and renewed blood flow is imperative.
Isenberg JS, Romeo MJ, Abu-Asab M, Tsokos M, Oldenborg A, Pappan L, Wink DA, Frazier WA, Roberts DD. Increasing survival of ischemic tissue by targeting CD47. Circulation Research 2007 Mar 16;100(5):712-20.
Kacsorowski DJ, Billiar TR. Targeting CD47: NO limit on therapeutic potential. Circulation Research 2007 Mar 16:100(5):602-603 (editorial).
Isenberg JS, Ridnour LA, Dimitry J, Frazier WA, Wink DA, Roberts DD. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. Journal of Biological Chemistry 2006 Sep 8;281(36):26069-80.
Funding from the National Institutes of Health supported this research.
Washington University School of Medicine’s full-time and volunteer faculty physicians also are the medical staff of Barnes-Jewish and St. Louis Children’s hospitals. The School of Medicine is one of the leading medical research, teaching and patient care institutions in the nation, currently ranked fourth in the nation by U.S. News & World Report. Through its affiliations with Barnes-Jewish and St. Louis Children’s hospitals, the School of Medicine is linked to BJC HealthCare.
Siteman Cancer Center is the only NCI-designated Comprehensive Cancer Center within a 240-mile radius of St. Louis. Siteman Cancer Center is composed of the combined cancer research and treatment programs of Barnes-Jewish Hospital and Washington University School of Medicine.