Some 15 million Americans have high blood pressure that can’t be controlled with medication, leaving them at high risk for early death, stroke, heart disease or kidney failure.
Researchers at Washington University School of Medicine in St. Louis are evaluating whether an investigational device can help these patients keep their blood pressure in check. Similar to a pacemaker, the iPod-sized device is implanted under the skin near the collarbone, with wires that carry electrical signals to nerve receptors along the carotid arteries in the neck. The signals activate the body’s own system for regulating blood pressure.
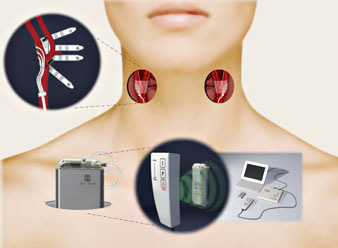
“The device is designed to fool the brain into thinking a person’s blood pressure is much higher than it really is,” says the lead investigator of a U.S. study evaluating the device, Marcos Rothstein, M.D., a professor of medicine at Washington University and medical director of dialysis services at Barnes-Jewish Hospital. “The brain, as the body’s central command center, responds by slowing the heart rate, relaxing the blood vessels, and filtering more salt and water from the kidneys – all of which lower blood pressure.”
At the recent annual meeting of the American College of Cardiology, Rothstein presented data from a small multi-center study of 38 patients with uncontrollable high blood pressure in whom the device was implanted. On average, their blood pressure was 183/105 despite taking antihypertensive medications. Two to three years after the device was implanted, they had reduced their systolic blood pressure (top number) by an average of 31 points and diastolic pressure by an average 21 points. “These are patients for whom no drugs have worked,” Rothstein says. “They had no other options.”
There were minimal side effects related to the device, the most common being temporary pain at the site where the device was implanted.
Rothstein, now leading a larger study, is seeking to enroll 300 U.S. patients, including 25 in St. Louis. The device is experimental but its maker, CVRx Inc., of Minneapolis, plans to submit data from the larger trial for U.S. approval.
To be eligible for the study, participants must be ages 18 – 75 and have blood pressure that has not responded to a combined regimen of the maximal dosages of at least two anti-hypertensives and a diuretic. Their systolic blood pressure must be consistently higher than 160 with this medication.
All participants will receive the medical device. Two-thirds will have the device turned on one month after it is implanted. As a comparison, the remaining one-third will have the device switched on seven months after it’s implanted. Participants will be randomly assigned to either group and must see Washington University physicians on a regular basis for checkups.
Medical tests and procedures related to the study, including the cost of the device and its implantation, will be provided free to patients.
For more information or to enroll in the trial, please contact Lisa Murphy, R.N., at (314) 747-3601 or lmurphy@DOM.wustl.edu.
The research is sponsored by CVRx.
Washington University School of Medicine’s 2,100 employed and volunteer faculty physicians also are the medical staff of Barnes-Jewish and St. Louis Children’s hospitals. The School of Medicine is one of the leading medical research, teaching and patient care institutions in the nation, currently ranked third in the nation by U.S. News & World Report. Through its affiliations with Barnes-Jewish and St. Louis Children’s hospitals, the School of Medicine is linked to BJC HealthCare.