Seeing inside: This whole-body MRI scanner, designed for clinical and research applications, offers high-resolution imaging of large anatomical areas and eliminates the need for patient repositioning during a scan. Members of the research team include Robert T. Naismith, MD, Anne H. Cross, MD, Sheng-Kwei (Victor) Song, PhD, and Robyn S. Klein, MD, PhD.
More than a century after multiple sclerosis (MS) was first recognized as a distinct pathologic disorder, its hallmark continues to be its frustrating unpredictability. Francis Clark, diagnosed with the autoimmune condition in high school, has learned to live with it. According to Clark, “MS is simply a fact of life.”
MS symptoms — inflammation of the optic nerve, loss of muscle strength and balance problems, to name just a few — can cause a wide range of problems. No two people have the same experience with MS and, as Clark points out, a flare-up of one or more symptoms can happen at any time, with varying recovery times.
“It seems to happen in a three-year cycle or close to it,” she says of her own disease. “Sometimes I get better very quickly, and sometimes it takes a long time. It can be very frustrating, not just for me, but also for my family. ”
But the veil of unpredictability is finally starting to give way. Neurologists, radiologists and others at the School of Medicine have teamed up to show that an imaging technique, diffusion tensor imaging (DTI), can help assess damage to the optic nerves from MS. Now they are working to apply the same technique to other MS lesions that lead to symptoms affecting a broad range of the body. The questions they are posing about MS may one day allow better prognostic information and contribute to identifying more effective treatments.
Scientists believe that MS, which affects an estimated 500,000 Americans, results from misdirected immune system attacks against the central nervous system.
“For many years prior to the advent of MR imaging, MS was a disease of exclusion,” says Anne H. Cross, MD, the Manny and Rosalyn Rosenthal–Dr. John L. Trotter chair in neuroimmunology and director of the John L. Trotter MS Clinic at Barnes-Jewish Hospital. “The doctor would first rule out a list of other potential diagnoses until MS was the only thing left. This would sometimes lead to misdiagnoses that made it harder to understand MS.”
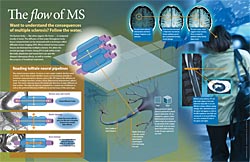
MRI has revolutionized the care of MS patients by allowing early and accurate diagnosis. However, standard MR imaging techniques didn’t help researchers reliably make a critical distinction for understanding, predicting and treating MS: When was MS damaging axons, the fibers leaving nerve cells that carry impulses from one nerve cell to another? And when was it harming only myelin, the protective sheath around the axons?
“When an axon loses its myelin, it can still carry information,” Cross explains. “It may not be able to transmit it as effectively, but it can still work. And there’s evidence that many people with MS may be able to regenerate their myelin.”
When an axon is lost, however, the prognosis is grim. No messages can be conveyed, and there is little evidence that the axon can regenerate.
Using a mouse model of MS, Cross collaborated with Sheng-Kwei (Victor) Song, PhD, associate professor of radiology, to see whether an experimental imaging technique using the MRI scanner, known as diffusion tensor imaging (DTI) could help to distinguish between myelin damage and axon loss. DTI uses MRI to track water diffusion in tissue.
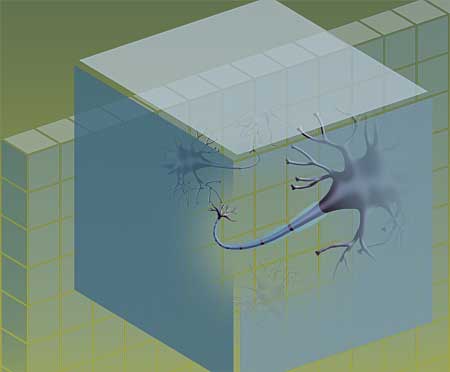
Song and Cross reasoned that MS inflammation and the damage it causes would likely alter water diffusion in the affected tissues. Song thought a new approach to interpreting the DTI results could be particularly useful: analyze water diffusion both down the length of a nerve axon (axial diffusion) and across the insulating myelin (radial diffusion).
“We were able to show that a decrease in axial diffusion was a very good bio-marker for axonal injury, and an increase in radial diffusion was a good marker for myelin damage,” says Song. “And we were able to correlate axonal injury with axon damage and increased likelihood of permanent disability.”
Next, Robert T. Naismith, MD, assistant professor of neurology, led an effort to apply the approach to human MS patients with inflammation of the optic nerve, which causes loss of vision, blurring or fogginess and pain in the affected eye. They found that DTI could be used to predict both the severity and permanence of damage from these episodes.
Now, with support from a new National Institutes of Neurological Disorders and Stroke grant, these scientists are applying DTI to other MS questions. “The optic nerve was our proof of concept, because it’s structurally a very simple tract with all the nerves going one direction, like a one-way street,” says Naismith. “Now we’re taking the technique into the brain and spinal cord, where there are numerous streets, including many that cross. Measuring damage and correlating it to dysfunction will be more complex as a result.”
Cross, Song and others are trying to use DTI to learn more about a phenomenon informally known as “black holes”: large, MS-induced lesions in the central nervous system that show up as dark spots on MRI scans.
“There’s so much tissue damage that the black holes can look almost like strokes,” Cross explains. “We’re trying to use DTI to help us predict which lesions, at early stages, will eventually develop into black holes. Patients with that type of MS lesion may need more aggressive treatments.”
Some data for the project will come from a recently completed clinical trial of a potential MS treatment that Cross conducted at Barnes-Jewish Hospital. The team is currently writing up their findings.
DTI also may help researchers understand MS at a more basic level. Robyn S. Klein, MD, associate professor of medicine, is using DTI to study a molecule her lab has linked to myelin regrowth. Her lab will block this molecule to see whether disabling the nerve cell’s ability to regrow the myelin sheath leads to axonal damage. She also plans to block individual molecules involved in inflammatory processes to see whether they can prevent the initial damage that occurs during MS flares.
“Being able to study these processes in live animals with DTI is extremely powerful,” Klein says. “By doing so, we can determine whether preventing early axonal injury with a drug is directly linked to eventual recovery from the flare-up.”
The same principle may work in clinical trials of new drug treatments in humans. Currently, such studies must wait weeks, months or longer to determine whether a new treatment has prevented permanent disability. If DTI provides quicker and more conclusive results, it will accelerate the drug development process.
Such knowledge is power, says MS sufferer Clark, recalling the days when her disease first began to manifest and she had yet to be diagnosed. “To have the right people working to find out what’s going on and to find the right way to treat MS is very helpful.”

This article appeared in the Fall 2009 issue of Washington University School of Medicine’s Outlook magazine.
Comments and respectful dialogue are encouraged, but content will be moderated. Please, no personal attacks, obscenity or profanity, selling of commercial products, or endorsements of political candidates or positions. We reserve the right to remove any inappropriate comments. We also cannot address individual medical concerns or provide medical advice in this forum.