Researchers at Washington University in St. Louis have developed algorithms to identify weak spots in tendons, muscles and bones prone to tearing or breaking. The technology, which needs to be refined before it is used in patients, one day may help pinpoint minor strains and tiny injuries in the body’s tissues long before bigger problems occur.
The research is available online Aug. 27 in the Journal of the Royal Society Interface, which publishes research at the nexus of the physical and life sciences.
“Tendons are constantly stretching as muscles pull on them, and bones also bend or compress as we carry out everyday activities,” said senior investigator Stavros Thomopoulos, PhD, professor of orthopaedic surgery. “Small cracks or tears can result from these loads and lead to major injuries. Understanding how these tears and cracks develop over time therefore is important for diagnosing and tracking injuries.”
To that end, Thomopoulos and his colleagues developed a way to visualize and even predict spots where tissues are weakened. To accomplish this, they stretched tissues and tracked what happened as their shapes changed or became distorted.
The paper’s first author, John J. Boyle, a graduate student in biomedical engineering, combined mechanical engineering fundamentals with image-analysis techniques to create the algorithms, which were tested in different materials and in animal models.
“If you imagine stretching Silly Putty or a swimming cap with a picture on it, as you pull, the picture becomes distorted,” Boyle said. “This allows us to track how the material responds to an external force.”
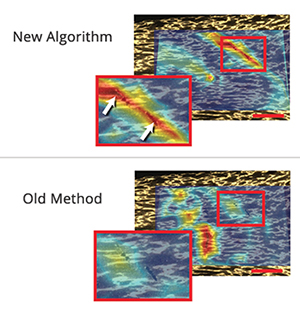
In one of the experiments described in the paper, Boyle sprayed a pattern of dots on plastic wrap, stretched it and tracked the dots.
“As you pull and stretch the plastic wrap, eventually tears begin to emerge,” he explained. “The new algorithm allowed us to find the places where the tears were beginning to form and to track them as they extended. Older algorithms are not as good at finding and tracking localized strains as the material stretches.”
In fact, one of the two new algorithms is 1,000 times more accurate than older methods at quantifying very large stretches near tiny cracks and tears, the research showed. And a second algorithm has the ability to predict where cracks and failures are likely to form.
“This extra accuracy is critical for quantifying large strains,” said Guy Genin, PhD, professor of mechanical engineering and co-senior investigator on the study. “Commercial algorithms that estimate strain often are much less sensitive, and they are prone to detecting noise that can arise from the algorithm itself rather than from the material being examined. The new algorithms can distinguish the noise from true regions of large strains.”
Thomopoulos, who also is a professor of biomedical engineering and of mechanical engineering, works with Genin to study the shoulder’s rotator cuff, a group of tendons and muscles that connect the upper arm to the shoulder blade. They want to learn why some surgeries to repair rotator cuff injuries ultimately fail. Their goal is to increase the odds that the tissue in the shoulder will heal following surgery, and they believe the new algorithms could help them get closer to that goal.
How soon the new algorithms could be used in patients depends on getting better images of the body’s tissues. Current imaging techniques, such as MRI and ultrasound, lack the required clarity and resolution.
Genin also explained that although the goal of the current study is to better understand how forces at work on human tissue cause injury and stress, the algorithms also could help engineers identify vulnerable parts of buildings and other structures. Our muscles and bones, he said, are influenced by the same strains that affect those structures.
“Whether it’s a bridge or a tendon, it’s vital to understand the ways that physical forces cause structures and tissues to deform so that we can identify the onset of failures and eventually predict them,” he said.
In the long run, they want to use the algorithms to prevent additional injuries following surgery to repair knees, shoulders and other tissues. They also said it may be possible some day to predict problems before they occur.
The group, which applied for a provisional patent earlier this year, hopes the algorithms will be useful to researchers in the medical and engineering fields.
This work was funded by the National Institute on Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (NIH) and the National Science Foundation (NSF). NIH grant number U01EB016422.
Boyle JJ, Kume M, Wyczalkowski MA, Taber LA, Pless RB, Xia Y, Genin GM, Thomopoulos S. Simple and accurate methods for quantifying deformation, disruption and development in biological tissues. Journal of the Royal Society Interface, vol. 11 (100) 20140685. http://dx.doi.org/10.1098/rsif.2014.0685, published online Aug. 27, 2014.
Washington University School of Medicine’s 2,100 employed and volunteer faculty physicians also are the medical staff of Barnes-Jewish and St. Louis Children’s hospitals. The School of Medicine is one of the leading medical research, teaching and patient-care institutions in the nation, currently ranked sixth in the nation by U.S. News & World Report. Through its affiliations with Barnes-Jewish and St. Louis Children’s hospitals, the School of Medicine is linked to BJC HealthCare.
The School of Engineering & Applied Science at Washington University in St. Louis focuses intellectual efforts through a new convergence paradigm and builds on strengths, particularly as applied to medicine and health, energy and environment, entrepreneurship and security. With 91 tenured/tenure-track and 40 additional full-time faculty, 1,300 undergraduate students, 750 graduate students and more than 23,000 alumni, we are working to leverage our partnerships with academic and industry partners — across disciplines and across the world — to contribute to solving the greatest global challenges of the 21st century.