Fuel cells could someday generate electricity for nearly any device that’s battery-powered, including automobiles, laptops and cellphones. Typically using hydrogen as fuel and air as an oxidant, fuel cells are cleaner than internal combustion engines because they produce power via electrochemical reactions. Since water is their primary product, they considerably reduce pollution.
One issue that impacts the lifetime of the fuel cell is the oxidation, or breakdown, of its central electrolyte membrane. The process leads to formation of holes in the membrane and can ultimately cause a chemical short circuit.
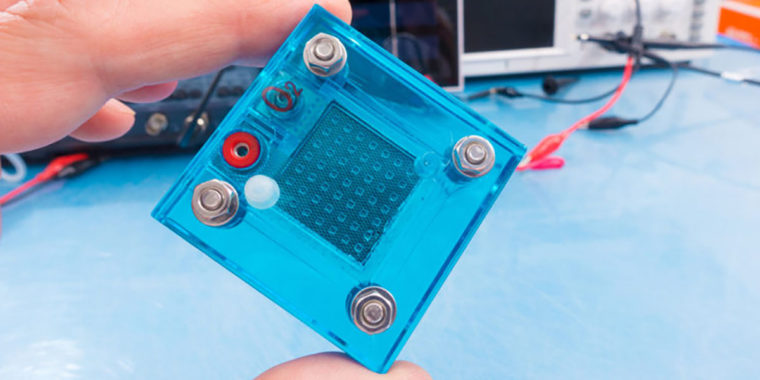
An engineering team at Washington University in St. Louis has developed a new way to take a look at the rate at which oxidation occurs. Using fluorescence spectroscopy inside the fuel cell, they are able to probe the formation of the chemicals responsible for the oxidation, namely free radicals, during operation. The technique could be a game changer when it comes to understanding how the cells break down, and designing mitigation strategies that would extend the fuel cell’s lifetime.

“If you buy a device — a car, a cell phone — you want it to last as long as possible,” said Vijay Ramani, the Roma B. and Raymond H. Wittcoff Distinguished Professor of Environment & Energy at the School of Engineering & Applied Science. “Unfortunately, components in a fuel cell can degrade, and it’s not an easy fix. What our new research does is really shed light on one of the modes by which these devices can fail, allowing us to figure out methods so we can improve the lifetime of devices that use these fuel cells.”
The research, published this summer in the journal ChemSusChem, is the first to utilize an in situ approach to examine the fuel cell’s inner membranes. A fluorescent dye is incorporated and used as a marker to ascertain the rate at which damaging free radicals are generated during operation.
“By using fluorescence spectroscopy in conjunction with an optical fiber, we can quantify the oxidative free radicals generated inside the fuel cell, which work to break down the membranes,” said Yunzhu Zhang, a doctoral candidate in Ramani’s lab, and study co-author.
Once they were able to observe the fuel cell’s inner workings, the researchers noticed that the weaker the light emitted from the fuel cell membrane, the greater the breakdown occurring from within.
“We can see this process occurring in real time,” said Shrihari Sankarasubramanian, a postdoctoral researcher who assisted with the project.
Until now, researchers examining fuel cell breakdown relied on the cell’s emissions to determine which chemical reactions might have been to blame for membrane breakdowns. They say this new approach allows them to focus on the factors taking place inside for a better assessment.
“Since the free radicals that cause the fuel cell membrane degradation are so short-lived, and the anion exchange membranes are so thin, our novel in situ approach is key to better study, understand and prevent the chemical breakdowns that is occurring during fuel cell operation,” said Javier Parrondo, a postdoctoral researcher and research co-author.
“The next step is to introduce antioxidant chemicals inside the fuel cell membranes, to see if they can reduce the rate at which these membranes break down,” Ramani said.
Comments and respectful dialogue are encouraged, but content will be moderated. Please, no personal attacks, obscenity or profanity, selling of commercial products, or endorsements of political candidates or positions. We reserve the right to remove any inappropriate comments. We also cannot address individual medical concerns or provide medical advice in this forum.