
In 2010, on the occasion of accepting the Lee C. Howley Sr. Prize for his pioneering arthritis research, Wayne M. Yokoyama, MD, told his audience that real advances in science do not come from the close application of previously known arcane facts to solve difficult problems. Rather, he said, science requires taking risks to venture completely beyond what is known, pursuing nature’s secrets that she is not inclined to reveal easily. “There is art to science,” according to Yokoyama, who holds the Sam J. and Audrey Loew Levin Chair for Research in Arthritis.Yokoyama’s entire career has been informed by the sort of imaginative leaps and perceptive risks that produce big insight. For more than 20 years, he has been intrigued by the function of natural killer (NK) cells, a component of the innate immune system initially thought to be involved in reining in tumors. Perhaps because they were poorly understood, NK cells were long thought to be of minor importance. By building on observations and following where experiments led — and in no small measure by trusting his intuition — Yokoyama has explored the uncommon molecular behavior of the cells, quelling controversies and disregarding the opinions of those who have thought him everything from mildly misguided to full-on crazy. Eventually, he showed that NK cells are high-functioning, sophisticated elements of the immune system that select their targets in a complex biochemical interaction and are vitally important in the body’s response (and even over-response) to a variety of invaders, including viruses.
The die was cast early on when Yokoyama, a rheumatologist, chose to pursue the molecular activity of NK cells, even though none of his colleagues believed that they had anything to do with arthritis or autoimmune disorders, the primary concerns of rheumatologists. “I thought we had a good clue,” he says, explaining that his lab, then at the University of California, San Francisco, had identified a protein on the surface of NK cells. It was the same protein first identified on T-cells, a critical part of the immune response and a topic of intense interest in immune system research.
One of the great good fortunes of Yokoyama’s career, he says, was that his division’s director at the time, the eminent rheumatologist Ira Goldstein, MD, continued to support the research, even though Yokoyama says that both would have admitted, if asked about a connection between NK cells and arthritis, that there was “none that I know of.”
After locating the gene for the interesting molecule, Yokoyama’s suspicion became stronger, because the gene belonged to a large cluster in the genome, and the great number of related molecules were expressed on NK cells, pointing to functional significance. “These proteins were receptors,” meaning their purpose was to bind to other molecules, Yokoyama says, “but receptors in name only, because we hadn’t yet identified what they bound to.”
Pursuing the protein’s targets, Yokoyama and his colleagues next made one of their fundamental discoveries: The NK-cell receptors bound to the same molecules that T-cells used to identify invaders. These ligand molecules — known as MHC class-1 molecules (for Major Histocompatability Complex class-1) — are found on nearly every cell of the body except red blood cells. Unique to each individual, they identify cells as part of the self, as friend not enemy. And when a cell becomes infected, their job is to display fragments of the foreign, pathological proteins from within the cell so that T-cells recognize the sick cell and eliminate it.
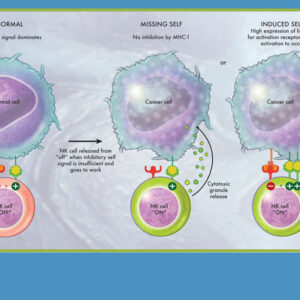
“That’s not the whole story, however,” Yokoyama says, “because NK cells also need to be activated.” He explains: “For an NK cell to defend the organism against infection, there needs to be no inhibition by MHC-1 plus activation by a second receptor.”
A major leap in thinking then was required to realize that NK cells bind to MHC-1 just as T-cells do, but work in the opposite fashion. “When an NK cell sees an MHC-1 molecule, it doesn’t note that there is a foreign peptide being displayed. Instead, its job is to patrol for self, and when it binds to MHC-1, the NK cell is shut off,” Yokoyama explains. “Only when the self signal is insufficient is the NK cell released from its ‘off’ condition and freed to do its work.” That’s vitally important information, because viruses have evolved to depress MHC-1 in their attempt to evade T-cells. So NK cells serve an important role as a fail-safe in the immune system, effective when another of the body’s defenses has been blinded by tricky infections.
“That’s not the whole story, however,” Yokoyama says, “because NK cells also need to be activated.” He explains: “For an NK cell to defend the organism against infection, there needs to be no inhibition by MHC-1 plus activation by a second receptor.”
The sophistication of NK-cell behavior revealed by this work sparked increased interest by investigators, and soon it was shown that the receptor in the mouse — Yokoyama’s research having been done in the murine model — and the corresponding human receptor were very different. The two proteins folded differently, and their distinct ends were reversed. Controversy ensued. “It appeared that someone was wrong,” Yokoyama says. To understand the apparent contradiction required another leap of imagination. “We had to think more broadly. The protein receptors couldn’t be more different, that’s true. But they work the same way and have the same purpose,” he says.
Geneticists call this turn of events “convergent evolution.” When a biological function is particularly crucial, various species may each evolve a discrete solution to arrive at the same result. It’s akin to the Roman alphabet and Asian logographic scripts arising independently: wildly different solutions to the same problem.
But, in the unending complexity of evolution’s schemes, even with that mystery resolved, there was still “a fly in the ointment,” Yokoyama says. “In humans or mice with no MHC-1, NK cells don’t run rampant as you would expect.” Yokoyama determined that is because first, NK cells must acquire the ability to function in a process he labels “licensing.” The same inhibitory receptor that later serves to recognize self also binds early in a cell’s life. The encounter changes a portion of NK cells into functionally competent immune system defenders, licensing them to work and regulating their activity. First comes the equivalent of a “standby” mode and then later the instruction to proceed with the job, almost like turning on a computer, then opening an application to do the work.
The licensing strategy Yokoyama uncovered was an entirely new biological process, and John Atkinson, MD, the Samuel Grant Professor of Medicine and acting head of rheumatology, says the work has “tackled and largely solved a major problem in innate immunology. Wayne’s experiments have placed molecular handles on the heretofore difficult issue of NK cell recognition of self.”
The early interaction between NK cells’ inhibitory receptors and MHC-1 molecules determines how effective any person’s NK cells will be. Those with the right combination of genes for receptors on NK cells and strong MHC-1 systems combat viruses — including HIV — more effectively. NK cells also play a role in the bone marrow transplantation essential for the treatment of leukemia. “Outcomes appear to be better if the recipient and the donor are mismatched for MHC-1, so that the donor cells both replace and attack the old, leukemic marrow,” Yokoyama explains.
“In science, you must follow the investigations and build on your observations, even when they seem unusual or meet resistance,” Yokoyama says.
Recently, others have learned that the cells also may contribute to certain types of arthritis, particularly juvenile rheumatoid arthritis. It appears that most patients with the disorder have poorly functioning NK cells. Could that condition be the basis for the disease? Yokoyama isn’t prepared to go that far, but he knows the evidence supports a continued effort to fully understand all of the interactions between NK cells and other molecules.
Twenty years of basic science investigations thus seem to be closing the circle. Yokoyama says he can imagine the pure science leading to a variety of potential therapies. It might be possible to stimulate NK cells to respond more powerfully. Or perhaps the inhibitory receptors that depress NK cell activity could be down-regulated. There’s even the potential to infuse cells with the gene for NK receptors, thereby increasing their numbers and effectiveness. Millions of immune system cells of several types circulate in our bloodstreams, searching for foreign pathogens. Designing a method to ramp up the potency of this one variety might result in powerful therapies with few side effects.
“In science, you must follow the investigations and build on your observations, even when they seem unusual or meet resistance,” Yokoyama says. “You may not initially see the connections, and your work may not be appreciated because it is so far from what is known. In this case, it is gratifying that an initial idea turns out to be relevant to human disease.”
Steve Kohler is a freelance writer based in Bonne Terre, Mo.

Comments and respectful dialogue are encouraged, but content will be moderated. Please, no personal attacks, obscenity or profanity, selling of commercial products, or endorsements of political candidates or positions. We reserve the right to remove any inappropriate comments. We also cannot address individual medical concerns or provide medical advice in this forum.