Defeating cancerous tumors by attacking healthy cells seems like an unusual strategy, but researchers at Washington University School of Medicine in St. Louis have shown the strategy to be effective against leukemia/lymphoma in mice.
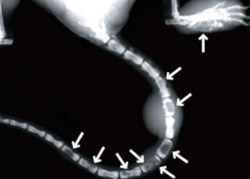
Led by Katherine N. Weilbaecher, M.D., assistant professor of medicine, the research group found that inhibiting normal bone-maintenance cells called osteoclasts not only prevented the mice’s cancer from spreading to their bones, it also slowed the growth of tumors in the body’s soft tissues.
“Tumor cells can mutate to overcome the treatments we use, but normal body cells won’t,” says Weilbaecher, an oncologist with the Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital. “And since cancer cells depend on the normal cells in our body for their survival, we can sometimes get at them by targeting more vulnerable host cells. In this case, by going after osteoclasts, we were able to affect tumor cells.”
The mice used in the study were developed in the laboratory of Lee Ratner, M.D., Ph.D., professor of medicine, of molecular microbiology and of pathology and immunology. The Ratner group introduced a gene called Tax into the mice’s genome. Having the Tax gene makes the mice very susceptible to T-cell leukemia/lymphoma, a blood cancer that also forms soft-tissue tumors and metastasizes to invade bones.
The Tax gene comes from human T-cell leukemia virus type 1 (HTLV-1), a virus that infects 10 to 20 million people worldwide and causes an aggressive form of adult T-cell leukemia/lymphoma in about 5 percent of infected people.
When the Tax-transgenic mice develop this cancer, their tumor cells secrete factors that activate osteoclasts. Osteoclasts are one of two types of cells that participate in the normal renewal of bone throughout an organism’s lifetime — osteoclasts break down old bone and osteoblasts build up new bone to maintain bone health.
By activating osteoclasts, the mice’s tumors cause excessive bone breakdown, and their bones develop holes that allow cancerous cells to enter and proliferate.
When the research group inhibited osteoclast activity, they saw that the mice were protected from tumor-associated bone destruction and from bone tumors. But interestingly, the mice also developed fewer tumors in other areas of the body. Their tumors grew more slowly, and the mice survived longer than mice without the Tax gene.

“Because cancer caused by HTLV-1 is known to cause bone destruction, when we introduced the Tax gene into the mice, we expected it to cause cancer and induce the production of proteins that stimulate osteoclasts to attack bone,” Weilbaecher says. “But we also found that when the activated osteoclasts start destroying bone, they release growth factors that feed back and encourage tumor growth. Inhibiting osteoclast activity reduced the production of tumor growth factors.”
One way the researchers blocked osteoclast activation was to administer a drug called bisphosphonate or zoledronic acid to the Tax-transgenic mice. Bisphosphonate targets bone and has been used for several years as an osteoporosis preventative in menopausal women because it interferes with the bone-degrading activity of osteoclasts.
“After menopause, the drop in estrogen levels allows osteoclasts to rev up their resorption of bone, and bisphosphonate helps slow that process,” Weilbaecher says. “We thought that breast cancer patients taking estrogen suppressors might have increased osteoclast activity that could encourage the growth of microscopic tumors in the bone marrow by releasing tumor growth factors. So we are also studying the effect of bisphosphonate on breast cancer metastasis and so far are seeing encouraging results.”
Weilbaecher and her colleagues will continue to study the Tax-transgenic mice to identify more precisely the factors that encourage tumor growth and the spread of cancer into bone.
Weilbaecher also is interested in the potential for affecting leukemia development in people who are infected with the HTLV-1 virus. Their study demonstrated that treating the leukemia/lymphoma-susceptible, Tax-transgenic mice with bisphosphonate altered the course of the leukemia/lymphoma, and Weilbaecher would like to see if bisphosphonate can also be used to intervene before HTLV-1-positive people develop the associated T-cell leukemia/lymphoma, which is resistant to current therapies.
Gao L, Deng H, Shao H, Hirbe A, Harding J, Ratner L, Weilbaecher K. HTLV-1 Tax transgenic mice develop spontaneous osteolytic bone metastases prevented by osteoclast inhibition. Blood, December 15, 2005;106(13):4294-4302.
Funding from the National Institutes of Health, a Pfizer/Washington University Biomedical Research grant and an Edward G. Mallinckrodt Jr. Foundation grant supported this research.
Washington University School of Medicine’s full-time and volunteer faculty physicians also are the medical staff of Barnes-Jewish and St. Louis Children’s hospitals. The School of Medicine is one of the leading medical research, teaching and patient care institutions in the nation, currently ranked third in the nation by U.S. News & World Report. Through its affiliations with Barnes-Jewish and St. Louis Children’s hospitals, the School of Medicine is linked to BJC HealthCare.
Siteman Cancer Center is the only NCI-designated Comprehensive Cancer Center within a 200-mile radius of St. Louis. Siteman Cancer Center is composed of the combined cancer research and treatment programs of Barnes-Jewish Hospital and Washington University School of Medicine.