Flick a switch on a dark winter day and your office is flooded with bright light, one of many everyday miracles to which we are all usually oblivious.
A physicist would probably describe what is happening in terms of the particle nature of light. An atom or molecule in the fluorescent tube that is in an excited state spontaneously decays to a lower energy state, releasing a particle called a photon. When the photon enters your eye, something similar happens but in reverse. The photon is absorbed by a molecule in the retina and its energy kicks that molecule into an excited state.
Light is both a particle and a wave, and this duality is fundamental to the physics that rule the Lilliputian world of atoms and molecules. Yet it would seem that in this case the wave nature of light can be safely ignored.
Kater Murch, assistant professor of physics in Arts & Sciences at Washington University in St. Louis, might give you an argument about that. His lab is one of the first in the world to look at spontaneous emission with an instrument sensitive to the wave rather than the particle nature of light, work described in the May 11th issue of Nature Communications.
His experimental instrument consists of an artificial atom (actually a superconducting circuit with two states, or energy levels) and an interferometer, in which the electromagnetic wave of the emitted light interferes with a reference wave of the same frequency.
In spontaneous emission, wave detection gives you more information than particle detection, as Kater Murch explains in this animation he drew and narrated.
This manner of detection turns everything upside down, he said. All that a photon detector can tell you about spontaneous emission is whether an atom is in its excited state or its ground state. But the interferometer catches the atom diffusing through a quantum “state space” made up of all the possible combinations, or superpositions, of its two energy states.
This is actually trickier than it sounds because the scientists are tracking a very faint signal (the electromagnetic field associated with one photon), and most of what they see in the interference pattern is quantum noise. But the noise carries complementary information about the state of the artificial atom that allows them to chart its evolution.
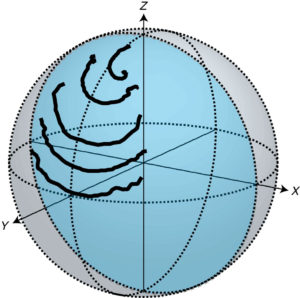
When viewed in this way, the artificial atom can move from a lower energy state to a higher energy one even as its follows the inevitable downward trajectory to the ground state. “You’d never see that if you were detecting photons,” Murch said.
So different detectors see spontaneous emission very differently. “By looking at the wave nature of light, we are able see this lovely diffusive evolution between the states,” Murch said.
But it gets stranger. The fact that an atom’s average excitation can increase even when it decays is a sign that how we look at light might give us some control over the atoms that emitted the light, Murch said.
This might sound like a reversal of cause and effect, with the effect pushing on the cause. It is possible only because of one of the weirdest of all the quantum effects: When an atom emits light, quantum physics requires the light and the atom to become connected, or entangled, so that measuring a property of one instantly reveals the value of that property for the other, no matter how far away it is.
Or put another way, every measurement of an entangled object perturbs its entangled partner. It is this quantum back-action, Murch said, that could potentially allow a light detector to control the light emitter.
“Quantum control has been a dream for many years,” Murch said. “One day, we may use it to enhance fluorescence imaging by detecting the light in a way that creates superpositions in the emitters.
“That’s very long term, but that’s the idea,” he said.
