If traditional drug delivery were a type of painting, it might be akin to paintball. With good aim, a majority of the paint ends on the bullseye, but it also drips and splashes, carrying streams of paint across the target.
If the drug needs to enter the bloodstream and circulate throughout your body for treating disease wherever it may be, this paintball-like delivery system may work. But it won’t work for targeted and precise drug delivery.
A more acute delivery approach would look more like “painting by numbers,” a technique that would allow precise delivery of a certain amount of drugs to an exact location. Researchers at the McKelvey School of Engineering and the School of Medicine at Washington University in St. Louis are developing the tools necessary for such a drug delivery system, which they call cavitation dose painting.

Their research was published online this week in Scientific Reports.
Using focused ultrasound with its contrast agent, microbubbles, to deliver drugs across the blood-brain barrier (FUS-BBBD), the research team, led by Hong Chen, assistant professor of biomedical engineering at McKelvey School of Engineering, and assistant professor of radiation oncology at the School of Medicine, was able to overcome some of the uncertainty of drug delivery.
This method takes advantage of the microbubbles expanding and contracting when they interact with the ultrasound, essentially pumping the intravenously-delivered drug to wherever the ultrasound is pointing.
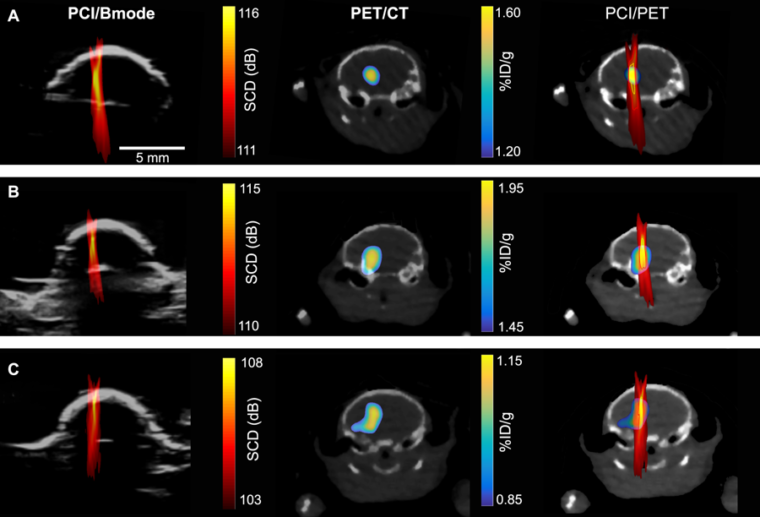
To determine where and how much of the drugs were being delivered, the researchers used nanoparticles tagged with radio labels to represent drug particles, then used positron emission tomography (PET) imaging to track their whereabouts and concentrations. They could then create a detailed image, showing where the nanoparticles were going and in what concentrations.
There’s one hitch, though.
“The problem is, PET imaging is expensive and associated with radioactive exposure,” Chen said.
So the team turned to passive cavitation imaging (PCI), an ultrasound imaging technique that has been under development by several groups for imaging the spatial distribution of microbubble cavitation, or the oscillation of microbubbles in the ultrasound field.
To determine whether PCI could also accurately determine the amount of drugs at a certain location, they correlated a PCI image with a PET image (which they knew can quantify the concentration of radioactive agents).
“We found there’s pixel by pixel correlation between the ultrasound imaging and the PET imaging,” said Yaoheng Yang, the lead author of this study and a second-year PhD student in the Department of Biomedical Engineering. The PCI image, therefore, can be used to predict where a drug goes and how much drug is there. Hence, she called the new technique cavitation dose painting.
Going forward, Chen believes this method could drastically change the way some drugs are delivered. Using cavitation dose painting in tandem with focused ultrasound will allow doctors to deliver precise amounts of drugs to specific locations, for example, targeting different areas of a tumor with exactitude.
“I think this cavitation dose painting technique in combination with focused ultrasound-enabled brain drug delivery opens new horizons in spatially targeted and modulated brain drug delivery,” Chen said.
She has recently received a $1.6 million grant from the National Institutes of Health (NIH)’s National Institute of Biomedical Imaging and Bioengineering to work on combining intranasal drug delivery and focused ultrasound (FUSIN) with this research.
The research team from Washington University School of Medicine included Xiaohui Zhang, postdoctoral research associate of radiology; Richard Laforest, associate professor of radiology; Yongjian Liu, associate professor of radiology; and Jeffrey F. Williamson, professor of radiation oncology. From McKelvey Engineering: Haoheng Yang; and Dezhuang Ye.
This work was in part supported by the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital (grant number MC-II-2017-661), American Cancer Society (grant number IRG-15-170-58), and the National Institutes of Health (NIH) grant R01MH116981 and R01EB027223.
The McKelvey School of Engineering at Washington University in St. Louis focuses intellectual efforts through a new convergence paradigm and builds on strengths, particularly as applied to medicine and health, energy and environment, entrepreneurship and security. With 96.5 tenured/tenure-track and 33 additional full-time faculty, 1,300 undergraduate students, 1,200 graduate students and 20,000 alumni, we are working to leverage our partnerships with academic and industry partners — across disciplines and across the world — to contribute to solving the greatest global challenges of the 21st century.