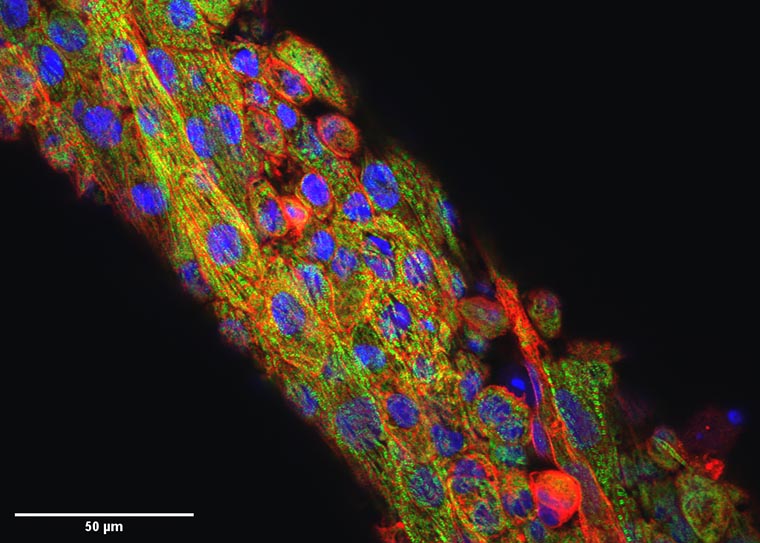
Researchers at Washington University in St. Louis have developed a new computational approach to removing movement in images of expanding and contracting heart cells and tissues. By computationally removing movement, the algorithm mimics a drug’s action in stopping the heart, without compromising cellular structure or tissue contractility.
Results of the research, led by Nathaniel Huebsch, an assistant professor of biomedical engineering, and Guy Genin, the Harold and Kathleen Faught Professor of Mechanical Engineering, both at the McKelvey School of Engineering, are published in the Sept. 11 Proceedings of the National Academy of Sciences.

“When the heart beats, the electrical signal goes through the tissue, and that triggers mechanical squeezing,” said Huebsch, who studies how mechanical cues affect heart development and disease. “In many cases, deadly arrhythmias happen because the electric pulse becomes decoupled from the mechanical squeezing. Our group leverages biomaterials and genetics to study how inherited cardiomyopathies develop, and we are very excited to have a tool that allows us, for the first time, to directly and non-invasively study electro-mechanical coupling in cardiomyocytes.”
The team’s algorithm mimics the action of blebbistatin, a drug that inhibits motion of heart muscle but also can negatively impact the underlying structure of these cells.
This so-called virtual blebbistatin was created from two algorithms: one that has been successful in estimating displacements for cardiac mapping; and another that uses the first to map evolving signals back to a stabilized image of the tissue. The approach allows researchers to directly monitor the coupling of calcium waves, membrane voltage and mechanical contraction beat by beat in heart tissue models, giving insight into diseases of the heart.
Read more on the McKelvey School of Engineering website.