Physicians treating patients with early-stage lung cancer face a conundrum: choosing potentially helpful yet toxic therapies such as chemotherapy, radiation or immunotherapy to knock out the cancer and lessen the risk of it spreading to the brain, or waiting to see if lung surgery alone proves sufficient. When up to 70% of such patients do not experience brain metastasis — the spread of cancer to the brain — the question arises: Who should receive additional aggressive treatments, and who can safely wait?
A new study led by Washington University School of Medicine in St. Louis could help physicians strike the right balance between proactive intervention and cautious monitoring for patients with early-stage lung cancer. The study, published March 4 in The Journal of Pathology, uses an artificial intelligence (AI) method to study patients’ lung biopsy images and predict whether the cancer will spread to the brain.
“There are no predictive tools available to help physicians when treating patients with lung cancer,” said Richard J. Cote, MD, the Edward Mallinckrodt Professor and head of the Department of Pathology & Immunology. “We have risk predictors that tell us which population is more likely to progress to more advanced stages, but we lack the ability to predict individual patient outcomes. Our study is an indication that AI methods may be able to make meaningful predictions that are specific and sensitive enough to impact patient management.”
Lung cancer is the leading cause of cancer death in the U.S. and worldwide. Most lung cancers are characterized as non-small cell lung cancers, which are largely, but not exclusively, caused by smoking. For early-stage cancer patients, tumors are confined to the lung, and surgery is recommended as a first line of treatment. Roughly 30% of such patients progress to advanced stages, when the cancer spreads to the lymph nodes and other organs. With the brain often affected first, such patients require additional treatments, including chemotherapy, targeted drug therapy, radiation therapy and/or immunotherapy. However, physicians have no way of knowing whose cancer will progress, so they frequently treat patients with aggressive therapies out of caution.
Cote worked with Ramaswamy Govindan, MD, the Anheuser Busch Endowed Chair in Medical Oncology and associate director of the oncology division at Washington University; Mark Watson, MD, PhD, the Margaret Gladys Smith Professor in the Department of Pathology & Immunology; and Changhuei Yang, PhD, a professor of electrical engineering, bioengineering, and medical engineering at the California Institute of Technology, to determine if AI could predict whether cancer will spread to the brain.
In diagnostic testing, a pathologist examines biopsied tissues under a microscope to identify cellular abnormalities that may hint at disease. Advanced technologies — such as AI — are being explored to replicate what a pathologist sees when making diagnoses but with greater accuracy, Cote explained.
A key question: Can AI detect abnormal features that a pathologist cannot?
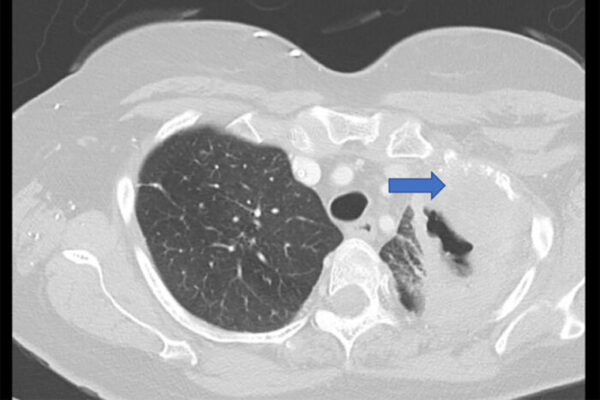
The researchers trained a machine-learning algorithm to predict brain metastasis using 118 lung biopsy samples from early-stage non-small cell lung cancer patients. Some of the patients developed brain cancer during a five-year monitoring period, and some did not and were in remission. Then the researchers tested the AI method on its ability to predict brain metastasis, and to identify patients who develop no metastasis, using 40 other patients’ lung biopsy samples.
The algorithm was able to predict the eventual development of brain cancer with 87% accuracy. In comparison, four pathologists who participated in the study were an average of 57.3% accurate. Importantly, the algorithm was highly accurate in predicting which patients would not develop brain metastasis.
“Our results need to be validated in a larger study, but we think there is great potential for AI to make accurate predictions and impact care decisions,” said Govindan, who treats lung cancer patients at Siteman Cancer Center, based at Barnes-Jewish Hospital and Washington University School of Medicine. “Systemic treatments such as chemotherapy, while effective in killing cancer cells, can also harm healthy cells and are not always the preferred treatment method for all early-stage cancer patients. Identification of patients who are likely to relapse in the brain may help us develop strategies to intercept cancer early in the process of metastasis. We think AI-based predictions could, one day, inform personalized treatments.”
The AI system evaluates tumors’ and healthy cells’ features, similar to how the human brain allows us to scan facial features for quick recognition of familiar faces. However, what the algorithm sees is unknown; the scientists are working to understand the molecular and cellular features that AI uses for its predictions. This knowledge could lead to the development of novel therapeutics and influence the design of imaging instruments optimized for the collection of data for AI.
“This study started as an attempt to find predictive biomarkers,” said Yang. “But we couldn’t find any. Instead, we found that AI has the potential to make predictions about cancer progression using biopsy samples that are already being collected for diagnosis. If we can get to a prediction accuracy that will allow us to use this algorithm clinically and not have to resort to expensive biomarkers, we are talking about significant ramifications in cost effectiveness.”
Zhou H, Watson M, Bernadt CT, Lin S, Lin CY, Ritter JH, Wein A, Mahler S, Rawal S, Govindan R, Yang C and Cote RJ. AI-guided histopathology predicts brain metastasis in lung cancer patients. The Journal of Pathology. March 4, 2024. DOI: 10.1002/path.6263
This work was supported by the National Cancer Institute of the National Institutes of Health (NIH), grant numbers 5R01CA182746 and U01CA233363; Washington University in St. Louis School of Medicine Personalized Medicine Initiative; Sensing to Intelligence, grant number 13520296; and the Heritage Research Institute for the Advancement of Medicine and Science at Caltech, grant number HMRI-15-09-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
About Washington University School of Medicine
WashU Medicine is a global leader in academic medicine, including biomedical research, patient care and educational programs with 2,900 faculty. Its National Institutes of Health (NIH) research funding portfolio is the second largest among U.S. medical schools and has grown 56% in the last seven years. Together with institutional investment, WashU Medicine commits well over $1 billion annually to basic and clinical research innovation and training. Its faculty practice is consistently within the top five in the country, with more than 1,900 faculty physicians practicing at 130 locations and who are also the medical staffs of Barnes-Jewish and St. Louis Children’s hospitals of BJC HealthCare. WashU Medicine has a storied history in MD/PhD training, recently dedicated $100 million to scholarships and curriculum renewal for its medical students, and is home to top-notch training programs in every medical subspecialty as well as physical therapy, occupational therapy, and audiology and communications sciences.
Originally published on the School of Medicine website