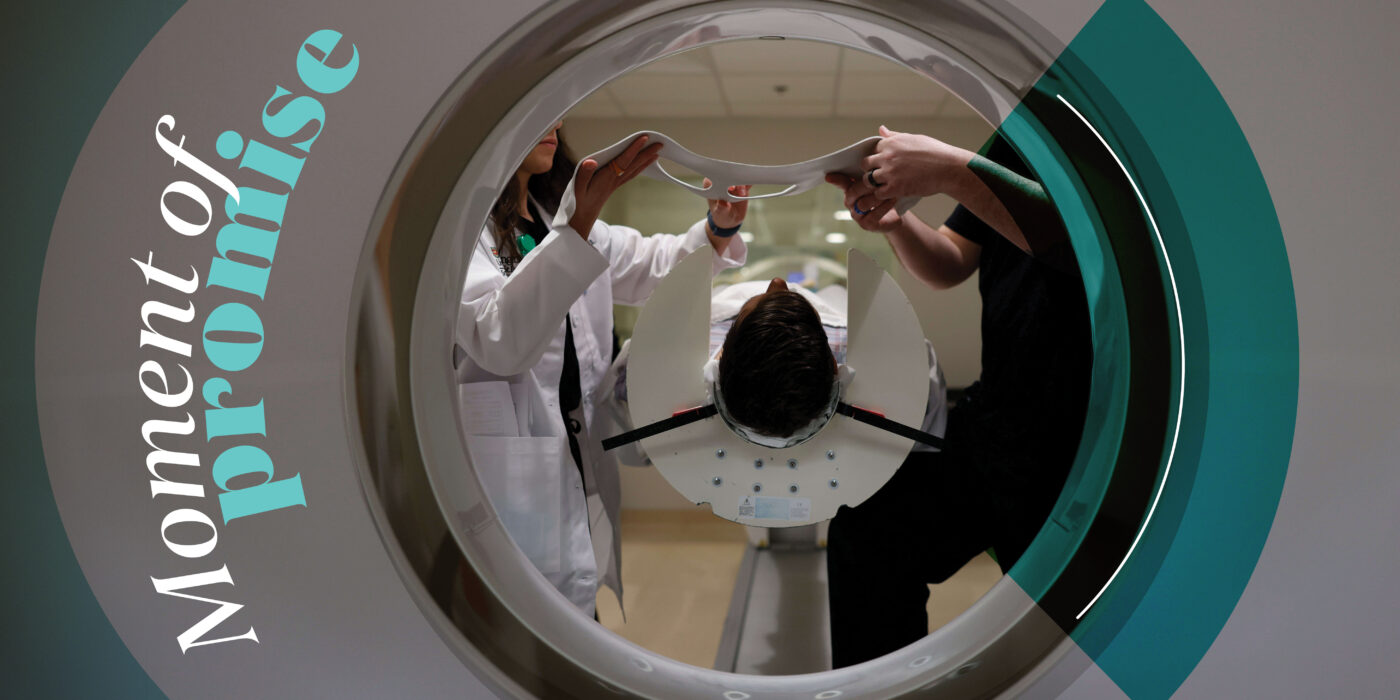
Washington University School of Medicine has always been a place for jaw-dropping medical advances, from pioneering innovations in radiology to the mapping of the human genome. And the pace of discovery continues to quicken. The university recently dedicated the new 609,000-square-foot Jeffrey T. Fort Neuroscience Research Building, which houses some 120 research teams — one of the highest concentrations of neuroscientists in the world. The researchers are committed to unlocking the mysteries of the human brain and tackling the intractable neurological and psychiatric diseases confronting humankind.
“We’re ranked No. 2 among U.S. medical schools in overall research funding from the National Institutes of Health (NIH) and No. 1 in NIH funding for neurology research,” says David H. Perlmutter, MD, executive vice chancellor for medical affairs, the George and Carol Bauer Dean of the School of Medicine and the Spencer T. and Ann W. Olin Distinguished Professor. “When I think about where Washington University can have a unique, indelible impact on human health, it is in the neurosciences. This is the next frontier of science, in which new technologies and a coalescence of great talent at this university can help us address human suffering.”
Throughout the rest of the story, Washington Magazine will introduce readers to some of the creative, collaborative work being done in this “next frontier of science” by expert researchers as well as everyday people and their families, who are putting everything on the line for a chance at cures.
The Alzheimer’s families
Through a landmark program started at Washington University School of Medicine, families with inherited, early-onset Alzheimer’s fight for a cure.
Christmas came early for the Redshaw children in 2023. On an ordinary day in early December, Ryan Redshaw was home with his three young children when several boxes were delivered to the house. The boxes contained toys, he discovered, so he handed them out. It wasn’t until his wife came home and expressed dismay that he realized his mistake.
“All the Christmas presents I had ordered came on the same day, and he opened all the boxes and gave them to the kids,” Liana Redshaw says. “This is what early-onset Alzheimer’s looks like for us. It’s not just his memory that’s affected, but his thought processes, his judgment. Most people would see a bunch of toys right before Christmas and think, ‘I have to put these away.’ But Ryan didn’t make the connection. And so, the kids got Christmas on a random Tuesday this year.”
Ryan Redshaw is a participant in the Dominantly Inherited Alzheimer Network-Trials Unit (DIAN-TU), a global clinical trial led by Washington University School of Medicine involving people with a rare, genetic form of Alzheimer’s disease. To participate in the trial, volunteers must have inherited a mutation that all but guarantees they will develop memory and thinking problems in their 50s, 40s or even 30s. Washington University researchers in DIAN-TU and the related observational study, DIAN, have played a crucial role in unraveling the complex biology of Alzheimer’s disease over the past 16 years.
Once thought to be an inescapable part of aging, Alzheimer’s was redefined as a disease of the brain in 1906 by German physician Alois Alzheimer. Throughout the 20th century, the deadly disease remained unpreventable and untreatable. But now, finally, things have begun to change. The first drug shown to change the course of the disease, lecanemab, sold under the brand name Leqembi, was approved by the Food and Drug Administration (FDA) in January 2023. Another promising drug is currently under FDA review. A diagnostic blood test, developed by researchers at WashU’s School of Medicine, is in clinical use. In short, scientists are on the cusp of transforming this unrelenting scourge into a manageable threat.
Ryan Redshaw was diagnosed with inherited Alzheimer’s disease at the age of 33. The disease is so rare at his age that doctors routinely mistake it for more common problems such as depression, substance abuse or schizophrenia. But the Redshaws knew that Alzheimer’s was a possibility. Redshaw’s mother, Melissa “Mysi” Giesecki, as well as her mother and brother, had all developed dementia in their 30s and 40s. Redshaw doesn’t know his family history beyond those three because his grandmother was adopted. Until his own symptoms emerged, he’d held out hope that the disease would pass him by.
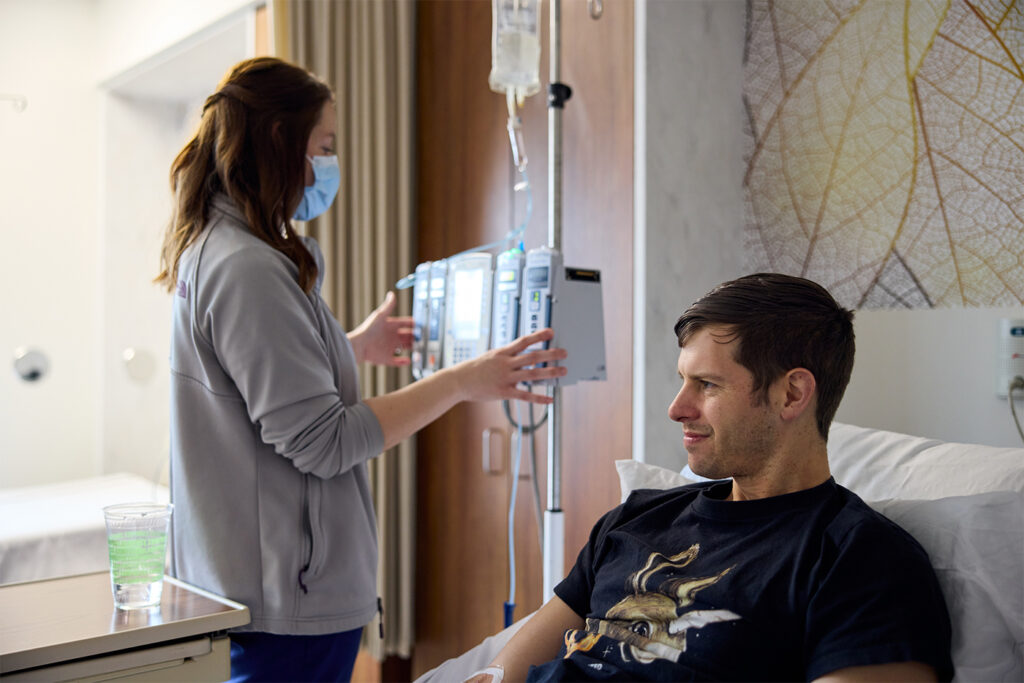
In the quarter century between Redshaw’s mother’s diagnosis and his own, the outlook for people with Alzheimer’s has gotten much brighter. We know tremendously more today about the disease and how to stop it, in no small part because of the work of WashU researchers.
Scientists first linked Alzheimer’s disease to the protein amyloid beta more than a century ago, when autopsies revealed plaques of the sticky protein cluttering the brains of people who died of the disease. The gene for amyloid was identified in 1987. A few years later, mutations were identified in that gene and two related genes that lead to the massive overproduction of amyloid and, consequently, early-onset Alzheimer’s disease. The Redshaw family carries a mutation in one of those three amyloid-related genes.
Around the time Redshaw’s mother started having memory problems in the late 1990s, scientists were beginning to suspect that cognitive decline was just the tail end of a very long process. Studying the adult children of Alzheimer’s patients, John C. Morris, MD, the Harvey A. and Dorismae Hacker Friedman Distinguished Professor of Neurology and then the director of WashU’s Charles F. and Joanne Knight Alzheimer Disease Research Center, discovered the characteristic amyloid plaques of Alzheimer’s in living people with no cognitive impairments. Morris also found that people with the highest amyloid levels were the most likely to develop memory and thinking problems later — evidence that amyloid builds up before cognitive symptoms emerge.
Discovering preclinical Alzheimer’s changed the game. It provided an opening for an intervention in which people on the road to dementia could still be pushed onto a safer path. All that doctors needed were (1) a way to find such people, and (2) a way to reduce the amyloid in their brains. In 2006, when Giesecki died, they had neither. Now, both are in reach.
Step No. 1: Find those at risk
After his mother’s death, Ryan Redshaw continued living with his stepfather in Florida until he finished high school. After graduation, he moved to Austin, Texas, for college, then returned to Florida to begin a career in marketing. Scientists now know that amyloid starts accumulating two decades or more before Alzheimer’s symptoms set in. As Redshaw began his adult life, the disease may have been taking hold already in his brain.
Meanwhile, in 2005, Randall J. Bateman, MD, the Charles F. and Joanne Knight Distinguished Professor of Neurology, started the Familial Adult Children Study at Washington University, to study families like the Redshaws that carry a mutation in one of the three amyloid-related genes. The study was a deep dive into their biology, using a technique Bateman co-invented called Stable Isotope Labeling Kinetics (SILK) to study amyloid production and clearance, along with cognitive testing, brain imaging, and analysis of blood and spinal fluid at frequent intervals. The goal was to learn more about the natural history of the disease, and particularly the preclinical stage. All members of such families would be invited to participate, regardless of mutation status. Those who did not inherit the mutation provided a natural comparison group for those who did, similar in age, genetics and lifestyle. In 2007, Morris started writing up a proposal to expand the Familial Adult Children Study into what would become DIAN, a nationwide — now global — network of centers to study such families.
For the families, it was the first time anyone had focused attention on finding the underlying causes of their disease in a systematic way. “There had never been a coordinated approach to understanding this form of Alzheimer’s disease,” Morris says. “Some families were unaware that there were other families like them. They thought they were the only ones.”
Isolation bred shame and secrecy, says Wendy Sigurdson, a clinical nurse coordinator who has been working with DIAN participants at Washington University from the beginning. “I remember one mother who came in with her son soon after we started. The father had died of early-onset Alzheimer’s years before. She said people had blamed her for her husband’s illness, telling her that she was not feeding him properly,” Sigurdson says. “It was so hard for these families when nobody knew what was going on.”
Morris received a grant from the National Institutes of Health in 2008 to fund DIAN, and he became the principal investigator and Washington University the lead site. Today, DIAN spans 40 sites across 15 countries and five continents, and it follows more than 500 family members globally. Of note, DIAN was conceived as a partnership between families and researchers. For example, four family members are seated on the steering committee. Further, DIAN has hosted conferences since 2015 to keep families informed about the progress of the research program and get their feedback.
The DIAN observational study and families such as the Redshaws are a major reason the first goal is close to realization. Mutation carriers usually develop symptoms around the same age as their parents. By studying people genetically destined to develop symptoms at a known age, DIAN researchers have been able to piece together the hidden sequence of molecular and cellular events that culminates in cognitive symptoms. Studies in other populations verified that people who develop Alzheimer’s at older ages follow the same sequence, just later in life.
This detailed understanding of how Alzheimer’s disease starts and progresses over three decades — in members of DIAN families as well as people with nongenetic forms of the disease — underpins the first generation of diagnostic tools capable of identifying people in early stages of the disease, when symptoms are mild and difficult to diagnose. Among those new diagnostic tools is a blood test developed by Bateman and a team of WashU scientists based on research in people with late-onset, nongenetic Alzheimer’s. The test received a “Breakthrough Device” designation from the FDA and was approved for clinical use in 2020. An updated version of the test not only identifies people with amyloid in their brains but pinpoints how far the disease has progressed.
By providing some of the scientific knowledge that makes early diagnosis feasible, the DIAN families have given the rest of us a priceless gift. After all, they don’t need any fancy tests to tell them they’re at risk of developing dementia. They live under the shadow of that risk every day.
Step No. 2: Treat them
“My patients care about thinking, about memories and about taking care of themselves,” says Bateman, the founding director of DIAN-TU, DIAN’s clinical trials unit (Bateman also succeeded Morris as principal investigator of DIAN). “The goal was always not only to understand the process, but to intervene. In medicine, the way we intervene starts with a clinical trial.”
The logic was this: If amyloid sets the whole pathological cascade in motion, then removing amyloid early, when symptoms are mild or undetectable, should cut the cascade short. For the DIAN families, this would be their first chance to participate in a clinical trial. They were routinely excluded from other Alzheimer’s trials, as they were deemed outliers who might skew the data. But for a prevention trial, they were ideal. Researchers knew who was going to develop cognitive symptoms and when, so it would be relatively easy to tell if the experimental treatment made a difference. Plus, there was already an international network set up that could help perform the trial.
Still, it was a hard sell. A prevention trial had never been conducted in Alzheimer’s disease. More than 100 treatment trials had been done, with a success rate of essentially zero. With so much uncertainty, Bateman concluded that their best chance of success lay in testing multiple drugs at once. He designed a novel trial platform with three drug arms and a shared placebo group. But this created a new problem: All drug trials have a single sponsor responsible for running the trial, normally a pharmaceutical company. With three drugs, there were three potential sponsors, none of which was willing to give up control to a competitor. Washington University was the only neutral party.
“It was really fortunate that the university leadership at the time — Chancellor Mark Wrighton, Provost Holden Thorp and others — backed this very innovative and, frankly, risky idea,” Bateman says. “And they did it for the right reasons. They said, ‘This is our mission. We should be trying to improve human health.’ If they hadn’t been willing to take that risk, DIAN-TU would have never existed.”

DIAN-TU launched in 2012 as the first Alzheimer’s prevention trial in the world, using two drugs that attack amyloid in different ways. A third drug was added in 2017, but it was discontinued the following year due to safety concerns. Participation required serious commitment: injections or IV drug infusions once every four weeks, plus annual brain scans, spinal taps, blood draws, and cognitive and clinical evaluations.
Even as DIAN-TU got underway, its foundational hypothesis — that targeting amyloid was the key to stopping Alzheimer’s — began to crack. Throughout the 2010s, amyloid drug after amyloid drug failed in clinical trials. Pharma companies started shutting down their amyloid drug-development programs. Funders stopped supporting amyloid approaches. Two noted Alzheimer’s researchers famously published a letter in The Wall Street Journal in 2015 decrying the “groupthink mentality” that compelled scientists to continue focusing on amyloid despite evidence the approach “performs at or worse than a placebo, coconut oil or marijuana.” The DIAN-TU’s top-line results, reported in 2020, were just as disappointing as other clinical trials of the time: No benefit was found for either drug in terms of cognitive function.
“People were somber, deflated,” Bateman says. “We tried so hard for so long, and we had nothing to show for it. And then, just a few years ago, we saw a glimmer of hope. Data started coming in from other trials that showed the amyloid hypothesis wasn’t dead after all. You see one trial, but it’s a small trial, with some benefit, and you think, ‘Maybe?’ And then there’s a second trial, and you think, ‘Wow, maybe this is something.’ And then the third trial is a big one, and by this time, everyone knows it’s working.”
“I started in Alzheimer’s research 40 years ago, and the advances of the past three years are beyond anything I had anticipated seeing in my career.”
John C. Morris, MD
On the heels of a frustrating and demoralizing decade, the Alzheimer’s community suddenly found itself with a win. The long-delayed success was the result of years of incremental improvements in Alzheimer’s clinical trials. Researchers at Washington University and elsewhere identified and developed molecular markers of disease that made it possible to screen potential participants more effectively; before such biomarkers were available, up to 30% of clinical trial participants probably didn’t have Alzheimer’s at all. In addition, trials shifted from enrolling people with more advanced symptoms to enrolling those with milder symptoms, as the disease’s natural history indicated that amyloid drugs likely would be more effective when begun sooner rather than later.
“I started in Alzheimer’s research 40 years ago, and the advances of the past three years are beyond anything I had anticipated seeing in my career,” Morris says. “Finally, we have something that benefits patients. I will be the first to admit that the amount of benefit doesn’t rise to the level of a cure, but it’s a beginning.”
Looking ahead
For the Redshaws, the tide also turned in the 2020s, but for the worse. “It’s hard to say when he started showing symptoms,” Liana Redshaw says. “For the past several years, we’ve been raising babies. There’s a lot of chaos and not a lot of sleep. It’s hard to tell ordinary mistakes from unusual mistakes.”
By the summer of 2023, however, it was clear something was not right. “I’d be dressing the kids, and I’d say to Ryan, ‘Could you go get some clothes?’ And he’d go and not come back. I’d find him doing something else,” Liana Redshaw says. “He just seemed off. He was distant when we’d always had a close relationship.”
Ryan Redshaw was diagnosed with Alzheimer’s last summer while his wife was pregnant with their third child. Despite the demands on two working parents raising a young family, the couple still makes time most nights, after the kids are in bed, to document their day on TikTok. It’s a way for them to strengthen their connection with each other, process their experiences and raise awareness of early-onset Alzheimer’s.
Redshaw is enrolled in the second iteration of DIAN-TU. A later, more thorough analysis of data from the first DIAN-TU revealed encouraging signs: One of the drugs improved molecular markers of disease and then in a follow-up study was found to delay symptom onset.
“One thing we learned from the amyloid trials is that you can’t expect to stop Alzheimer’s completely by attacking just one part of it. We must start thinking about additive therapies.”
B. Joy Snider, MD, PhD
“One thing we learned from the amyloid trials is that you can’t expect to stop Alzheimer’s completely by attacking just one part of it,” says B. Joy Snider, MD, PhD, a professor of neurology at the medical school who runs the Alzheimer’s clinical trials. “We must start thinking about additive therapies. At some point, we’ll probably be giving two or three or even more drugs that may vary depending on the stage of disease and other factors.”
The second DIAN-TU trial is called Tau NexGen because it includes experimental therapies targeting tau, another brain protein that also plays a critical role in Alzheimer’s disease. The first arm of Tau NexGen, the one Ryan Redshaw is in, combines the FDA-approved amyloid drug lecanemab with an investigational tau drug. Both drugs are made by Eisai Co., Ltd., a Japanese pharmaceutical company that collaborates closely with Washington University on Alzheimer’s drug discovery projects. Two more arms featuring combinations of amyloid and tau drugs are planned.
The DIAN network also is preparing to launch a third trial. Called the Primary Prevention trial and led by Eric McDade, DO, a professor of neurology, this trial is aimed at young adults who aren’t expected to develop symptoms for 15 years or more.
“We can detect signs of pathology up to two decades before symptoms arise,” McDade says. “That opens this window of opportunity to alter the amyloid pathway at the very earliest stage. The goal is to stop the disease process before it really gets started and prevent symptoms from ever emerging.” If it works, the Primary Prevention trial will break the curse that has haunted generations of DIAN families.
Wherever these studies lead, though, they won’t get there fast enough for some members of DIAN families. At enrollment, participants are asked to donate their brains upon their deaths. Nearly everyone agrees.
“I have one young gentleman who has been in the studies and has begun to deteriorate. It’s only a matter of time now,” Sigurdson says. “I’m going to have to call his mother and work with her to arrange for his brain donation. This call is always so, so tough. But for the participants, it’s their final gift — a wonderful, precious gift — and we appreciate it so much.”
After the brain is examined by the neuropathology team, Bateman will call the family to give them the results and thank them again for all they have done for science. It is one last recognition of the enormous sacrifices they have made to provide the crucial clues that could end Alzheimer’s forever — not just for their own children, but for everyone.
Are clues to Alzheimer’s hidden in our bowels?
WashU Medicine researchers explore the gut-brain connection.
From a bacterium’s point of view, a person is a walking, talking ecosystem. Tens of trillions of bacteria, representing 1,000 or more different species, live on and in the human body. We couldn’t live without our microscopic companions. They help digest food, provide vitamins, modulate immune responses and ward off harmful bacteria and fungi. The chemical compounds they release as byproducts of their metabolism influence, directly or indirectly, every organ in the human body.
Especially the brain. Doctors have long been aware of a special link between gut health and brain health. Digestive issues are common among people with neurological conditions including Alzheimer’s disease, Down syndrome and autism, and people with gastrointestinal conditions such as irritable bowel syndrome are at increased risk of psychiatric symptoms such as depression and anxiety.
“I can’t tell you how often I have a patient come in and say, ‘I’m having all these digestive problems,’” says neurologist Beau M. Ances, MD, PhD, the Daniel J. Brennan, MD Professor of Neurology. “I’m never surprised because there’s probably a connection.”
Ances co-led one of a complementary pair of studies published last year that provided key evidence of the close linkage between Alzheimer’s disease and the community of bacteria that live in the gut, known as the gut microbiome. The findings open the door to new ways of addressing Alzheimer’s via the gut, such as by using stool samples to identify people at risk before symptoms arise or treating patients with probiotics to reshape their gut microbiomes.
The findings also add to the mounting evidence that the gut microbiome plays a role in every aspect of how our brains work, from development to normal function to disease and degeneration. As a global leader in the science of both the microbiome and the brain, the Washington University School of Medicine stands at the forefront of efforts to understand how our bowels influence the health of our brains.

Jeffrey I. Gordon, MD, the Dr. Robert J. Glaser Distinguished University Professor and director of the Edison Family Center for Genome Sciences and Systems Biology, has been called the “father of the microbiome.” His work on the microbiome’s role in obesity and malnutrition has revolutionized our understanding of human biology, implicating the gut’s microbial residents in orchestrating healthy growth and development when these communities work well, and in causing disease when they do not. Many of his discoveries were made using specialized mouse models that allow researchers to measure the impact of gut microbial communities on the rest of the body. For a study published in January 2023, Gordon teamed up with David M. Holtzman, MD, the Barbara Burton and Reuben M. Morriss III Distinguished Professor and now the director of the Charles F. and Joanne Knight Alzheimer Disease Research Center at WashU Medicine, to use such mouse models to investigate how gut bacteria affect the brain. Their collaboration revealed that gut bacteria — partly by producing compounds such as short-chain fatty acids — affect the behavior of immune cells throughout the body, including ones in the brain that can damage brain tissue and exacerbate neurodegeneration in conditions such as Alzheimer’s disease.
In Ances’ study, published in June 2023, he collaborated with Gautam Dantas, PhD, the Conan Professor of Laboratory and Genomic Medicine and an expert on disruptions to the microbiome, to investigate how the microbiome differs in people at various stages of Alzheimer’s disease. Scientists already knew that the gut microbiomes of people with symptomatic Alzheimer’s differ from the microbiomes of healthy people of the same age. But Ances and Dantas showed that the microbiome was already significantly different in the earliest stage of the disease — after brain changes have begun but before cognitive symptoms become apparent.
Taken together, the two studies indicate that microbiome changes are closely linked to, and may even contribute to, the brain changes of Alzheimer’s disease. The two pairs of researchers are now working together to figure out whether the microbiome changes of Alzheimer’s disease are a cause or a result of the brain changes. They are taking stool samples from people with symptomatic Alzheimer’s disease and introducing them into mice lacking gut bacteria to see whether the gut bacteria trigger Alzheimer’s-like brain changes in the mice.
“That gives us an opportunity,” Dantas continues. “Envision a future where we’d have a very simple stool test to identify people with disease or at risk of disease. And then we could treat them with therapies that promote a healthier microbiome or that mimic healthy changes to the microbiome. There is so much potential for new ways of promoting human health that come from understanding the gut-brain connection.”
“The microbiome shouldn’t be thought of as an accessory to the human body; it’s an organ,” Dantas says. “Like any other organ, it interacts with and influences the rest of the body, and it is integral to normal development and health. But unlike other organs, the microbiome is fundamentally malleable. We can change it in ways that we cannot do with any other organ.