People with severe Parkinson’s disease or other neurological conditions that cause intractable symptoms such as uncontrollable shaking, muscle spasms, seizures, obsessive thoughts and compulsive behaviors are sometimes treated with electric stimulators placed inside the brain. Such stimulators are designed to interrupt aberrant signaling that causes the debilitating symptoms. The therapy, deep-brain stimulation, can provide relief to some people. But in others, it can cause side effects such as memory lapses, mood changes or loss of coordination, without much improvement of symptoms.
Now, a study from researchers at Washington University School of Medicine in St. Louis may help explain why the effects of deep-brain stimulation can vary so much – and points the way toward improving the treatment. The stimulators typically are implanted in structures known as the thalamus and the basal ganglia that are near the center of the brain. These structures, the researchers found, serve as hubs where the neurological networks that control movement, vision and other brain functions cross paths and exchange information. Each person’s functional networks are positioned a bit differently, though, so electrodes placed in the same anatomical spot may influence different networks in different people – alleviating symptoms in one but not in another, the researchers said.
The findings are published Dec. 10 in Neuron.
“The sites we target for deep-brain stimulation were discovered serendipitously,” said co-author Scott Norris, MD, an assistant professor of neurology and of radiology who treats patients with movement disorders. “Someone had a stroke or an injury in a specific part of the brain, and suddenly their tremor, for example, got better, and so neurologists concluded that targeting that area might treat tremor. We’ve never really had a way to personalize treatment or to figure out if there are better sites that would be effective for more people and have fewer side effects.”
What neurosurgeons lacked were individualized maps of brain functions in the thalamus and basal ganglia. These structures connect distant parts of the brain and have been linked to neurological and psychiatric conditions such as Parkinson’s disease, Tourette’s syndrome and obsessive-compulsive disorder. But their location deep inside the brain means that mapping is technically challenging and requires enormous amounts of data.
Nico Dosenbach, MD, PhD, an assistant professor of neurology and the study’s senior author – along with co-first authors Deanna Greene, assistant professor of psychiatry and of radiology, and Scott Marek, PhD, a postdoctoral researcher, and other colleagues – set out to create individual maps of the functional networks in the basal ganglia and thalamus. Such maps, they reasoned, might provide clues to why people with neurological and psychiatric conditions exhibit such a wide range of symptoms, and why electrodes placed in those structures produce variable results.
“Deep-brain stimulation is a very invasive treatment that is only done for difficult, severe cases,” said Greene, who specializes in Tourette’s syndrome. “So it is difficult to grapple with the fact that such an invasive treatment may only help half the people half the time.”
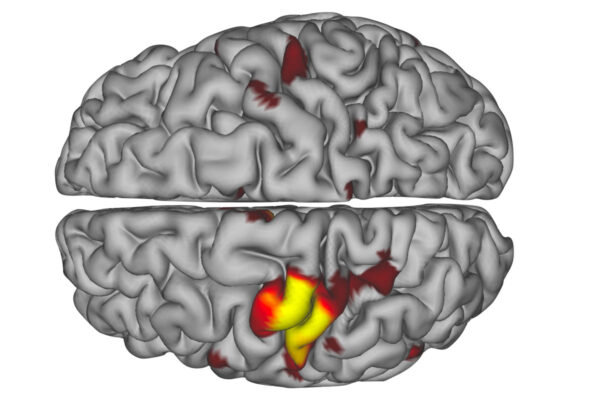
Using data from a group of Washington University scientists who scanned themselves at night as part of the so-called Midnight Scan Club, the researchers analyzed 10 hours of MRI brain scan data on each of 10 individuals. From this, they created 3D maps color-coded by functional network for each individual. One of the functional networks is devoted to vision, two relate to movement, two involve paying attention, three relate to goal-directed behaviors, and the last network is the default network, which is active when the brain is at rest.
The researchers discovered that each functional network followed its own path through the deep structures of the brain, intermingling with other networks at defined meeting spots. Some of these spots – such as the motor integration zone, where a movement and a goal-directed network come together – were located in much the same place in all 10 people. The locations of other networks and their points of intersection varied more from person to person.
“I showed a neurosurgeon where we’d found the motor integration zone, and he said, ‘Oh, that’s where we put the electrodes for essential tremor, and it always works,’” said Dosenbach, who is also an assistant professor of occupational therapy, of pediatrics and of radiology. People with essential tremor experience uncontrollable shaking. “That’s interesting because there isn’t a lot of variability among people in terms of where the motor integration zone is located. So then we looked at a spot they target to treat Parkinson’s disease. We saw that there was a great deal of variation across people in terms of what functional networks are represented there, and deep-brain stimulation is only about 40% to 50% successful there.”
The findings suggest that the outcome of deep-brain stimulation may reflect how successfully a neurosurgeon taps into the correct functional network – and avoids tapping into the wrong one.
“Historically, our understanding of deep-brain stimulation has been based on averaging data across many people,” Norris said. “What this study suggests is that a particular patient may do better if the wire is placed in relation to their personal functional brain map rather than in context of the population average. A personalized functional map – as opposed to an anatomical map, which is what we use today – could help us place a wire in the exact place that would provide the patient with the most benefit.”
The researchers now are studying the relationship between the locations of an individual’s functional networks and the outcomes of deep-brain stimulation to identify the networks that provide relief when stimulated, and those that cause side effects. In the future, the researchers hope to dig deeper into the networks with therapeutic effects, looking for other spots that might provide even better results than the traditional sites of electrode placement.
“A lot of what I do is basic science – understanding how the brain works,” Dosenbach said. “But now we can make a map and give it to neurosurgeons and potentially improve treatment for these devastating conditions. We still have to prove the hypothesis that deep-brain stimulation outcomes are linked to functional networks, and translation will take time, but this really could make a difference in people’s lives.”