A neuroscientist’s neon pink arm cast led him and fellow researchers at Washington University School of Medicine in St. Louis to discover previously undetected neuronal pulses in the human brain that activate after an immobilizing illness or injury.
The pulses appeared on MRI scans used to measure brain activity of the neuroscientist and, later, two additional adults whose arms were in casts. The researchers compared those MRI images with scans of the scientists before and after their arms were put in casts.
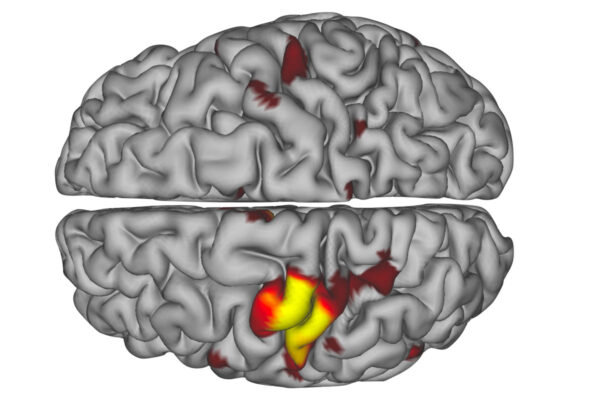
The scans showed that the brain’s main circuits responsible for movement in specific areas of the body disconnected within 48 hours of a person wearing a cast that encumbered movement in such an area. Also during this time, “disuse pulses” emerged to maintain neural activity and allow the main motor circuits to reactivate if and when mobility was restored through physical therapy.
The findings, published online June 16 in Neuron, offer clues to how the brain’s billions of neurons — cells that transmit nerve impulses — can rewire and restore pathways after injury or illness. Understanding just what is behind this resiliency may lead to new therapies for people with broken limbs or recovering from strokes or other immobilizing conditions.
“Many scenarios exist in neurology in which a person doesn’t use an arm or a leg and, consequently, related brain circuits for an extended period of time,” said senior author Nico Dosenbach, MD, PhD, assistant professor of neurology. “In offering the best care to patients, it’s important to understand specifically what changes occur in brain function. Accurate understanding and mapping of these circuits may lead to advancements in treating patients who have lost use of their limbs.”
In 2015, Dosenbach — also an assistant professor of occupational therapy, of pediatrics, of radiology and of biomedical engineering — wore the pink cast for two weeks despite the fact he had no injury requiring one. He aimed to collect high-quality data using brain-imaging techniques to evaluate neural networks that control movement.
Many of the patients Dosenbach treats at St. Louis Children’s Hospital suffer from conditions that limit mobility and cause them to favor one side of the body. A common treatment is constraint-induced movement therapy, also known as forced-use therapy, which immobilizes the dominant arm with a cast, forcing the child to use the impaired arm.
“My goal was a better understanding of what my patients experience during therapy, although I acknowledge it’s more difficult for them because of their disabilities,” Dosenbach said.
He also wanted to pinpoint a timeline of when individual neural changes occur. Commonly, scientists gather MRI data from dozens of people and average it. “But I did not want to do that because everyone’s brain is anatomically different, and when MRI data are averaged, it all blurs together,” Dosenbach said.
So Dosenbach decided to wear a fiberglass cast on his dominant, right arm. It stretched from his fingertips to just below his shoulder. It was pink, the favorite color of his daughter, Maike, then age 2.
He wore it during the hot, humid summer. It itched. It was awkward. He had to learn how to change a diaper with one hand.

Every day, he arose predawn to lie stiffly for 30 minutes for a resting-state functional MRI. He did this for the two weeks his arm was in the cast, as well as for the two weeks before and after.
During the six weeks, Dosenbach also wore accelerometers on both wrists to track the motor strength of his arms while performing basic tasks such as writing and moving objects.
“It wasn’t terrible, just unpleasant,” Dosenbach recalled. “But immediately, I noticed my right arm got worse, and my left hand got stronger. It was much faster than any of us expected.”
The MRI data showed that brain changes occurred within 48 hours. Additionally, the researchers measured a decrease in grip strength in his right arm — from 124 pounds of force to 90 during the two weeks he wore the cast.
“Once my cast was removed, my right hand began to grow stronger,” Dosenbach said. “My left returned to its former role, too.”
Surprised by such resiliency, Dosenbach and the study’s first author, Dillan Newbold, an MD-PhD student, conducted the same experiment on two “crazy-in-a-good-way” scientists — one who wore a fluorescent yellow cast decorated in doodles, the other a forest green cast that recalled childhood memories of camping.
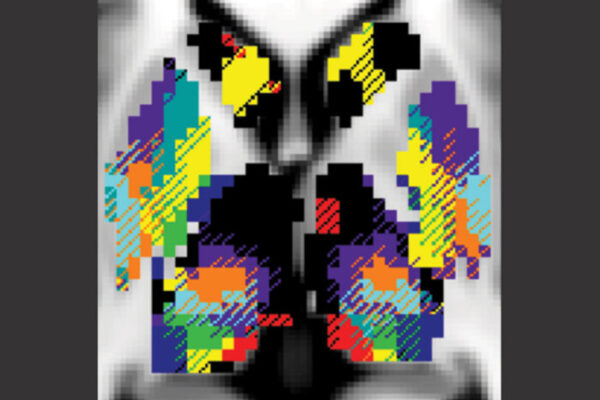
Using a resting-state functional MRI scan, the researchers identified and measured the precise regions in each individual’s brain that controlled each casted arm, examining more than 20 hours of recordings for each person. These techniques allowed the researchers to discover and characterize the pulses.
Their MRI data nearly mirrored Dosenbach’s. The findings indicated that disuse of each arm caused affected neurons to disconnect from the rest of the brain’s motor system within two days. Newbold’s analysis revealed that throughout the time the casts were worn, spontaneous pulses maintained activity in the disused circuits until the neurons began firing again when mobility was regained.
“Finding the spontaneous pulses was incredible,” Newbold said. “People can be motionless, but their neurons seem to protect the brain from completely disengaging when it’s not being used. More research is needed, but this was the most exciting part of the study because of the clinical implications.”