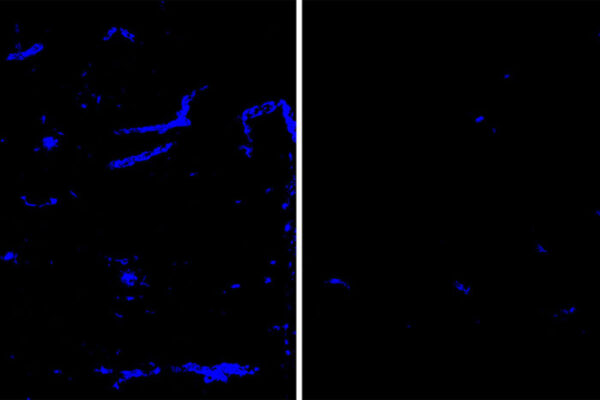
A worldwide clinical trial aimed at finding treatments for Alzheimer’s disease has expanded to include investigational drugs targeting a harmful form of the brain protein tau. The trial, known as the Dominantly Inherited Alzheimer Network Trials Unit (DIAN-TU) and led by Washington University School of Medicine in St. Louis, launched in 2012 as the first prevention trial for Alzheimer’s disease. Originally focused on amyloid-based therapies, it was funded by the National Institute on Aging (NIA) of the National Institutes of Health (NIH) in 2013.
Amyloid plaques in the brain are the first sign of Alzheimer’s disease. Such plaques start accumulating up to two decades before cognitive symptoms such as memory loss and confusion arise. For decades, Alzheimer’s researchers have searched for drugs to reduce or remove amyloid plaques as a way to treat the disease. Only recently have researchers begun developing drugs to target tau, which forms tangles in the brain that become detectable just before Alzheimer’s symptoms arise. The tangles are thought to be toxic to neurons, and their spread through the brain foretells the death of brain tissue and cognitive decline.
An experimental drug — an antibody called E2814 that recognizes the microtubule binding region (MTBR) of tau, developed by the pharmaceutical company Eisai Co. Ltd. — targets such tangles. It is the first of three planned tau drugs to be selected for evaluation in the DIAN-TU. The trial aims to determine whether such drugs reduce tau tangles and the damage caused by them, thereby slowing or stopping the progress of Alzheimer’s disease.
The addition of three new tau drug arms to the DIAN-TU is supported by grants expected to total $105 million from the NIA, $7 million each from the Alzheimer’s Association and GHR Foundation, and additional funding support from FBRI.
“As we’ve learned more about how Alzheimer’s develops, it has become clear that amyloid and tau both play critical roles in disease progression,” said principal investigator Randall J. Bateman, MD, director of DIAN-TU and the Charles F. and Joanne Knight Distinguished Professor of Neurology at Washington University. “Our prior studies show that some anti-amyloid drugs have positive biological effects in the brain. We will now test multiple different anti-tau drugs to determine if and how targeting tau can slow or stop the progression of Alzheimer’s disease.”
The DIAN-TU trial is the first trial aimed at identifying drugs to prevent or slow Alzheimer’s in people who are nearly certain to develop the disease due to genetic mutations. People who inherit such mutations tend to develop symptoms at around the same age their affected parents did, often in their 50s, 40s or even 30s. While devastating for families, such mutations allow researchers to identify and study people in the earliest stages of the disease before their behavior and memory begin to change.
Three trial arms were previously launched on the DIAN-TU trial platform to evaluate experimental drugs targeting amyloid. One, gantenerumab, reduced molecular markers of disease and slowed neurodegeneration in a phase 2/3 clinical trial. These promising results led the researchers to launch an exploratory open-label extension of the trial. Participants in both the drug and placebo groups have been offered the drug, and the researchers continue to monitor their progress.
The new arm of the DIAN-TU trial will evaluate an antibody that recognizes and binds to a part the MTBR region of the tau protein, which forms the major component of tau tangles.
“Eisai is pleased to participate in the groundbreaking Dominantly Inherited Alzheimer Network Trials Unit. We are hopeful that this study will generate important insights about our anti-microtubule binding region tau antibody, E2814, as well as benefit patients living with this devastating form of Alzheimer’s disease,” said Lynn Kramer, MD, chief clinical officer of Eisai’s Neurology Business Group. “As part of our human health-care mission, we are committed to making a difference for patients, their families and health-care professionals across the globe.”
Although the trial focuses on people with rare mutations, drugs that are successful in this population would be promising candidates for preventing or treating the forms of Alzheimer’s that occur more commonly in older adults. The destructive molecular and cellular processes in the brain are similar in all types of the disease.
“DIAN-TU is a prime example of how the research community, spurred by collaboration and federal funding, has built the capacity and put into motion clinical trials for emerging and promising drugs for Alzheimer’s,” said Richard J. Hodes, MD, director of the NIA. “The DIAN-TU studies, along with hundreds of other NIA-funded clinical trials, are advancing the field toward our national goal of discovering effective treatments and prevention strategies.”
The other two drug arms are expected to launch in 2022 and 2023. Although the specific experimental drugs have not been selected, the DIAN-TU research team plans to choose from two classes of tau drugs that act in different ways: by targeting other mechanisms of tau pathology, including small molecule drugs that inhibit tau aggregation; and genetic treatments that reduce the production of tau protein. The three arms of the trial will share one placebo group to maximize the number of people receiving the drugs.
“The Alzheimer’s Association is dedicated to funding and accelerating research in the pursuit of effective therapies for Alzheimer’s disease and all dementia, and that is why we’re expanding our support of the Dominantly Inherited Alzheimer’s Network Trials Unit,” said Maria C. Carrillo, PhD, Alzheimer’s Association chief science officer. “The field of Alzheimer’s research is maturing, so it’s crucial that studies like DIAN-TU build on that advancement and move promising treatments into clinical trials.”
The new drug arm builds on the well-developed infrastructure of the DIAN-TU platform and resources of the DIAN-TU network. Members of families with such mutations can enroll in a so-called “cognitive run-in” at any of 35 DIAN-TU sites across 13 countries. During the cognitive run-in, participants undergo cognitive tests and positron emission tomography (PET) brain scans for tau tangles to establish a baseline so they can be promptly enrolled in a drug arm once it is cleared to begin. The E2814 tau drug arm is planned to start in September, and participants will be treated and followed for at least two years.
The new tau drug arm will enroll participants from DIAN-TU families. Participants will be less than 10 years younger than the expected age of symptom onset or have developed symptoms fewer than 10 years ago. Participants will participate in regular cognitive tests and brain scans. Their cerebrospinal fluid and blood also will be analyzed for molecular signs of Alzheimer’s disease.
“As a platform trial, DIAN-TU plays a critical role in Alzheimer’s prevention by testing a variety of mechanisms,” said Fred Miller, GHR Foundation’s chief operating officer and Alzheimer’s program lead. “We are excited to join public and private partners in supporting the expanding scope of DIAN-TU as it begins testing tau therapies. Together with DIAN-TU families, we are reimagining what’s possible in our shared goal to prevent dementia caused by Alzheimer’s disease.”
The primary endpoint is a slowing of tau accumulation in the brain in symptomatic participants, as seen on PET brain scans. The researchers also will look at a specific kind of tau — phosphorylated tau 217 — in the cerebrospinal fluid of pre-symptomatic people. Bateman and colleagues have shown that levels of phosphorylated tau 217 in the cerebrospinal fluid rise steadily as the disease silently advances before symptom onset. A secondary endpoint will be a slowing of the rise in levels of this form of tau.
If these primary and secondary endpoints are positive, the trial will be extended for another two years to assess whether the drug slows or stops cognitive decline.
The DIAN-TU tau drug arms are primarily funded by NIH grants R01AG068319 and R01AG053267. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Washington University School of Medicine’s 1,500 faculty physicians also are the medical staff of Barnes-Jewish and St. Louis Children’s hospitals. The School of Medicine is a leader in medical research, teaching and patient care, ranking among the top 10 medical schools in the nation by U.S. News & World Report. Through its affiliations with Barnes-Jewish and St. Louis Children’s hospitals, the School of Medicine is linked to BJC HealthCare.