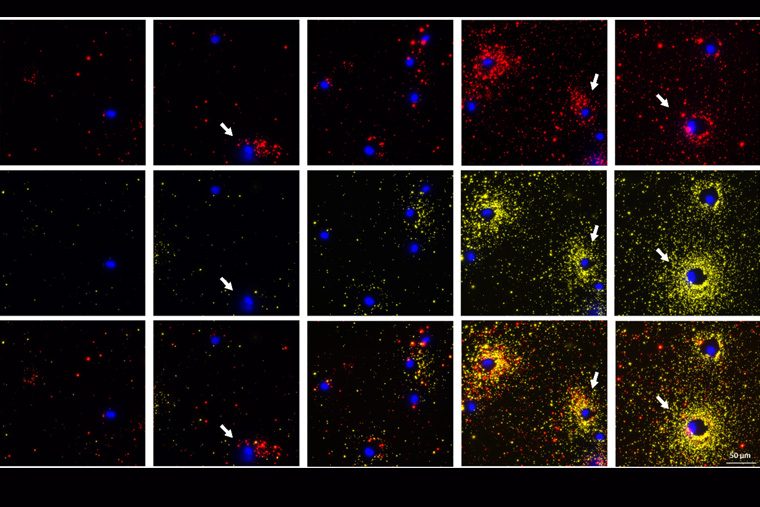
We have recently witnessed the stunning images of distant galaxies revealed by the James Webb telescope, which were previously visible only as blurry spots. Washington University in St. Louis researchers have developed a novel method for visualizing the proteins secreted by cells with stunning resolution, making it the James Webb version for visualizing single cell protein secretion.

The researchers, led by Srikanth Singamaneni, the Lilyan & E. Lisle Hughes Professor of Mechanical Engineering & Materials Science at the McKelvey School of Engineering, and Anushree Seth, a former postdoctoral scholar in Singamaneni’s lab, developed the FluoroDOT assay, which they introduced in a paper Aug. 5 in the journal Cell Reports Methods. The highly sensitive assay is able to see and measure proteins secreted by a single cell in about 30 minutes.
In collaboration with researchers at Washington University School of Medicine and other universities, they found that the FluoroDOT assay is versatile, low cost and adaptable to any laboratory setting and has the potential to provide a more comprehensive look at these proteins than the widely used existing assays. Biomedical researchers look to these secreted proteins for information on cell-to-cell communication, cell signaling, activation and inflammation, among other actions, but existing methods are limited in sensitivity and can take up to 24 hours to process.

What makes the FluoroDOT assay different from existing assays is that it uses a plasmonic-fluor, a plasmon-enhanced nanolabel developed in Singamaneni’s lab that is 16,000 times brighter than conventional fluorescence labels and has a signal-to-noise ratio nearly 30 times higher.
“Plasmonic-fluors are composed of metal nanoparticles that serve as antenna to pull in the light and enhance the fluorescence emission of molecular fluorophores, thus making it an ultrabright nanoparticle,” Singamaneni said.
This ultrabright emission of plasmonic-fluor allows the user to see extremely small quantities of secreted protein, which they are unable to do in existing assays, and measure the high-resolution signals digitally using the number of particles, or dot pattern, per cluster, or spot, using a custom-built algorithm. In addition, it doesn’t require special equipment. Singamaneni and his collaborators first published their work with the plasmonic-fluor in Nature Biomedical Engineering in 2020.
The patent-pending plasmonic fluor technology is licensed by the Office of Technology Management at Washington University in St. Louis to Auragent Bioscience LLC.
“Using a simple fluorescence microscope, we are able to simultaneously image a cell along with the spatial distribution of the proteins secreted around it,” said Seth, who had worked in Singamaneni’s lab and is now a principal scientist in cellular applications for Auragent Bioscience. “We saw interesting secretion patterns for different cell types. This assay also enables concurrent visualization of two types of proteins from individual cells. When the multiple cells are subjected to the same stimuli, we can distinguish the cells that are secreting two proteins at the same time from the ones that are only secreting one protein or are not secreting at all.”

To validate the technology, the team used proteins secreted from both human and mouse cells, including immune cells infected with Mycobacterium tuberculosis.
One of the collaborators and co-authors, Jennifer A. Philips, MD, PhD, the Theodore and Bertha Bryan Professor in the departments of Medicine and Molecular Microbiology and co-director of the Division of Infectious Diseases at the School of Medicine, has used the FluoroDOT assay in her lab.
“When Mycobacterium tuberculosis infects immune cells, those cells respond by secreting important immune proteins, called cytokines,” Philips said. “But not all cells respond to infection the same way. The FluoroDOT assay allowed us to see how individual cells in a population respond to infection — to see which cells are secreting and in which direction. This was not possible with the older technology.”
This research was supported with funding from the National Science Foundation, the National Institutes of Health (R21CA236652, R21EB030171, 2R01_AI087682, R01_304AI130454, R56AI14732).
The plasmonic fluor technology is licensed by the Office of Technology Management at Washington University in St. Louis to Auragent Bioscience LLC and has a patent pending. Jingyi Luan, Jeremiah J. Morrissey and Srikanth Singamaneni are co-founders and shareholders of Auragent Bioscience LLC.
The McKelvey School of Engineering at Washington University in St. Louis promotes independent inquiry and education with an emphasis on scientific excellence, innovation and collaboration without boundaries. McKelvey Engineering has top-ranked research and graduate programs across departments, particularly in biomedical engineering, environmental engineering and computing, and has one of the most selective undergraduate programs in the country. With 140 full-time faculty, 1,387 undergraduate students, 1,448 graduate students and 21,000 living alumni, we are working to solve some of society’s greatest challenges; to prepare students to become leaders and innovate throughout their careers; and to be a catalyst of economic development for the St. Louis region and beyond.