Interrupting nerve signals to the liver can prevent diabetes and hypertension in mice, according to scientists at Washington University School of Medicine in St. Louis. The finding is reported in the February issue of the journal Cell Metabolism.
 |
|
Researchers Clay Semenkovich and Carlos Bernal-Mizrachi explain the vagus nerve’s role in diseases such as hypertension and diabetes.
|
The research team surgically removed the vagus nerve in mice and found the procedure prevented or reversed the development of insulin resistance and high blood pressure in mice primed to develop these disorders through treatment with glucocorticoids.
“So at least in mice, we’ve shown we can prevent the development of diabetes and hypertension by interrupting vagal nerve signaling,” says senior investigator Clay F. Semenkovich, M.D., professor of medicine and of cell biology and physiology. “We don’t know whether the same will hold true for humans, but we think somehow altering vagal nerve activity could provide a novel approach for treating these common metabolic disorders.”
Previously, the research team had shown that a nuclear receptor called PPAR-alpha (Ppara) was necessary for the induction of both diabetes and hypertension when mice were treated with glucocorticoids, also known as steroids.

“Mice that can’t make Ppara don’t develop diabetes or hypertension in response to glucocorticoids,” says Semenkovich, who also is chief of the Division of Endocrinology, Metabolism and Lipid Research. “The use of steroids is very common in medicine. People with asthma, arthritis, organ transplants and others rely on those steroid drugs, and many of them go on to develop insulin resistance that can advance to diabetes and hypertension.”
But in these most recent experiments, the researchers showed that both Ppara and the vagus nerve seem to play important roles in the development of these disorders.
“If the vagus nerve has been surgically removed, the mice won’t develop diabetes or hypertension in response to glucocorticoids, even if they have Ppara,” says first author Carlos Bernal-Mizrachi, M.D., an assistant professor of medicine in the Division of Endocrinology, Metabolism and Lipid Research. “The process seems to be mediated by communication between the liver cells, the liver branch of the vagus nerve and its signals to the brain.”
Actually, the vagus nerve communicates with just about everything. Its name is taken from the Latin word meaning “wanderer.” Early neuroanatomists chose the name because it seemed whenever they looked at an organ in the body, they also found fibers from the vagus. It extends from the base of the brain, through the chest where it innervates part of the heart. It also sends nerve signals to other internal organs, including the liver, and eventually connects to the intestine. In these studies, however, the researchers were interested mainly in the connection between the vagus nerve coming from the liver and its communication with the brain.
When mice are treated with glucocorticoids, Ppara in the liver communicates with the vagus nerve, which signals the brain. Then the brain uses the vagal pathway to feed back instructions to the liver and kidneys. The brain instructs the liver to increase glucose production and the kidney to alter fluid metabolism, elevating blood pressure.
The same sort of process can occur in people who are obese. Semenkovich says a modest elevation of glucocorticoids is associated with obesity. Those elevated levels can initiate Ppara activity in the liver, which then will communicate with the vagus nerve to signal the brain and, in turn the brain will signal the liver and kidneys, contributing to diabetes and hypertension.
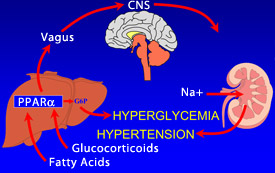
“We think obesity is probably initiating a similar process to the one we’ve interrupted in the mice,” says Semenkovich. “An environmental influence — such as treatment with glucocorticoids or excess caloric intake that causes obesity — engenders a signal started by Ppara, which then is transmitted from the liver, along the vagus nerve.”
That cascade of communication along the vagal nerve pathway has made the investigators think that they may be able to help people with diabetes and hypertension by interrupting normal vagal signaling. And there may be a ready-made population to study because many people already have surgically implanted devices that alter the signaling of the vagus nerve.
Some people with seizure disorders and treatment-resistant depression already have implanted electrodes that stimulate the vagus nerve to help alleviate their symptoms. Semenkovich believes the new mouse study suggests a similar approach might help people with insulin resistance or hypertension. They plan to follow patients who already have stimulators to see if signals from the stimulators affect susceptibility to diabetes, insulin resistance or hypertension.
“We used surgery to interrupt all signaling from the vagal nerve pathway,” Bernal-Mizrachi says. “But it might actually be possible to change very specific signaling patterns to provide benefit to people who are at risk for hypertension or diabetes.”
Some available drugs might be able to attack the problem in other ways. A class of medications called fibrate drugs can modulate the activity of Ppara. Those drugs are used to lower triglycerides and to elevate levels of HDL (good) cholesterol. Some studies have indicated the drugs provide a modest benefit, but other studies have suggested that such drugs might be harmful. So for now, the researchers are focusing more on the potential of the vagus nerve.
“I would argue that you can clearly produce a major impact by stimulating this nerve because it carries signals to so many organs,” Semenkovich says. “We know the vagal pathway can influence seizures, depression and other disorders. This study suggests it affects diabetes and hypertension, too.”
Bernal-Mizrachi C, Xiaozhong L, Yin L, Knutsen RH, Howard MJ, Arends JJA, DeSantis P, Coleman T, Semenkovich CF. An afferent vagal nerve pathway links hepatic PPARα activation to glucocorticoid-induced insulin resistance and hypertension. Cell Metabolism, vol. 5:2, pp. 91-102, Feb. 2007 DOI 10.1016/j.cmet.2006.12.010
This work was funded by grants from the National Institutes of Health and by an American Diabetes Association mentor-based postdoctoral fellowship.
Washington University School of Medicine’s full-time and volunteer faculty physicians also are the medical staff of Barnes-Jewish and St. Louis Children’s hospitals. The School of Medicine is one of the leading medical research, teaching and patient care institutions in the nation, currently ranked fourth in the nation by U.S. News & World Report. Through its affiliations with Barnes-Jewish and St. Louis Children’s hospitals, the School of Medicine is linked to BJC HealthCare.