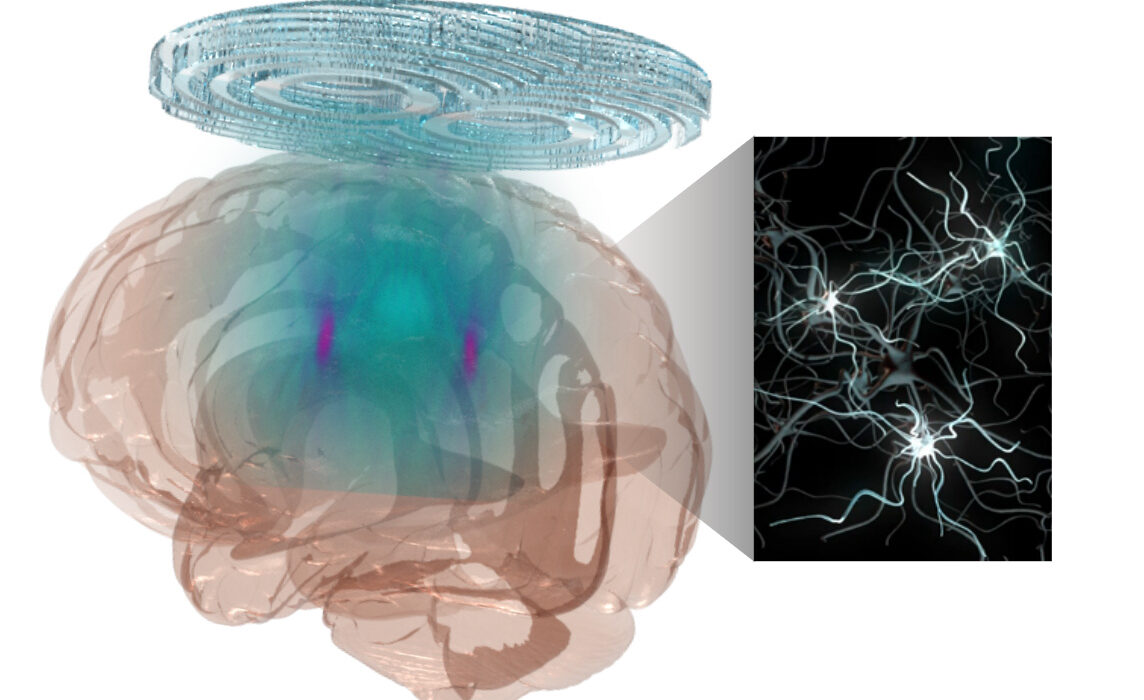
Human brain diseases, such as Parkinson’s disease, involve damage in more than one region of the brain, requiring technology that could precisely and flexibly address all affected regions simultaneously. Researchers at Washington University in St. Louis have developed a noninvasive technology combining a holographic acoustic device with genetic engineering that allows them to precisely target affected neurons in the brain, creating the potential to precisely modulate selected cell types in multiple diseased brain regions.
Hong Chen, an associate professor of biomedical engineering at the McKelvey School of Engineering and of neurosurgery at the School of Medicine, and her team created AhSonogenetics, or Airy-beam holographic sonogenetics, a technique that uses a noninvasive wearable ultrasound device to alter genetically selected neurons in the brains of mice. Results of the proof-of-concept study were published in Proceedings of the National Academy of Sciences June 17.
AhSonogenetics brings together several of Chen’s group’s recent advances into one technology. In 2021, she and her team launched Sonogenetics, a method that uses focused ultrasound to deliver a viral construct containing ultrasound-sensitive ion channels to genetically selected neurons in the brain. They use low-intensity focused ultrasound to deliver a small burst of warmth, which opens the ion channels and activates the neurons. Chen’s team was the first to show that sonogenetics could modulate the behavior of freely moving mice.
In 2022, she and members of her lab designed and 3D-printed a flexible and versatile tool known as an Airy beam-enabled binary acoustic metasurface that allowed them to manipulate ultrasound beams. She also is developing Sonogenetics 2.0, which combines the advantage of ultrasound and genetic engineering to modulate defined neurons noninvasively and precisely in the brains of humans and animals. AhSonogenetics brings them together as a potential method to intervene in neurodegenerative diseases.
“By enabling precise and flexible cell-type-specific neuromodulation without invasive procedures, AhSonogenetics provides a powerful tool for investigating intact neural circuits and offers promising interventions for neurological disorders,” Chen said.

Sonogenetics gives researchers a way to precisely control the brain, while airy-beam technology allows researchers to bend or steer the sound waves to generate arbitrary beam patterns inside the brain with a high spatial resolution. Yaoheng (Mack) Yang, a postdoctoral research associate who earned a doctorate in biomedical engineering from McKelvey Engineering in 2022, said the technology gives researchers three unique advantages.
“Airy beam is the technology that can give us precise targeting of a smaller region than conventional technology, the flexibility to steer to the targeted brain regions, and to target multiple brain regions simultaneously,” Yang said.
Chen and her team, including first authors Zhongtao Hu, a former postdoctoral research associate, and Yang, designed each Airy-beam metasurface individually as the foundation for wearable ultrasound devices that were tailored for different applications and for precise locations in the brain.
Chen’s team tested the technique on a mouse model of Parkinson’s disease. With AhSonogenetics, they were able to stimulate two brain regions simultaneously in a single mouse, eliminating the need for multiple implants or interventions. This stimulation alleviated Parkinson’s-related motor deficits in the mouse model, including slow movements, difficulty walking and freezing behaviors.
The team’s Airy-beam device overcomes some of the limits of sonogenetics, including tailoring the design of the device to target specific brain locations, as well as incorporating the flexibility to adjust target locations in a single brain.
Hu said the device, which costs roughly $50 to make, can be tailored in size to fit various brain sizes, expanding its potential applications.
“This technology can be used as a research platform to speed neuroscience research because of the capability to flexibly target different brain regions,” Hu said. “The affordability and ease of fabrication lower the barriers to the widespread adoption of our proposed devices by the research community for neuromodulation applications.”
Hu Z, Yang Y, Gong Y, Chukwu C, Ye D, Yue Y, Yuan J, Kravitz AV, Chen H. Airy-beam holographic sonogenetics for advancing neuromodulation precision and flexibility. Proceedings of the National Academy of Sciences June 17, 2024, DOI: 10.1073/pnas.2402200121.
Funding for this research was provided by the National Institutes of Health (R01NS12846, R01MH116981, UG3MH126861, R01EB027223, R01EB030102).
The design file for the Airy-beam holographic transducer is available on GitHub: https://github.com/ChenUltrasoundLabWUSTL/AiryBeam_Lens_Design.
Originally published on the McKelvey Engineering website

Comments and respectful dialogue are encouraged, but content will be moderated. Please, no personal attacks, obscenity or profanity, selling of commercial products, or endorsements of political candidates or positions. We reserve the right to remove any inappropriate comments. We also cannot address individual medical concerns or provide medical advice in this forum.