Electrostatic capacitors play a crucial role in modern electronics. They enable ultrafast charging and discharging, providing energy storage and power for devices ranging from smartphones, laptops and routers to medical devices, automotive electronics and industrial equipment. However, the ferroelectric materials used in capacitors have significant energy loss due to their material properties, making it difficult to provide high energy storage capability.
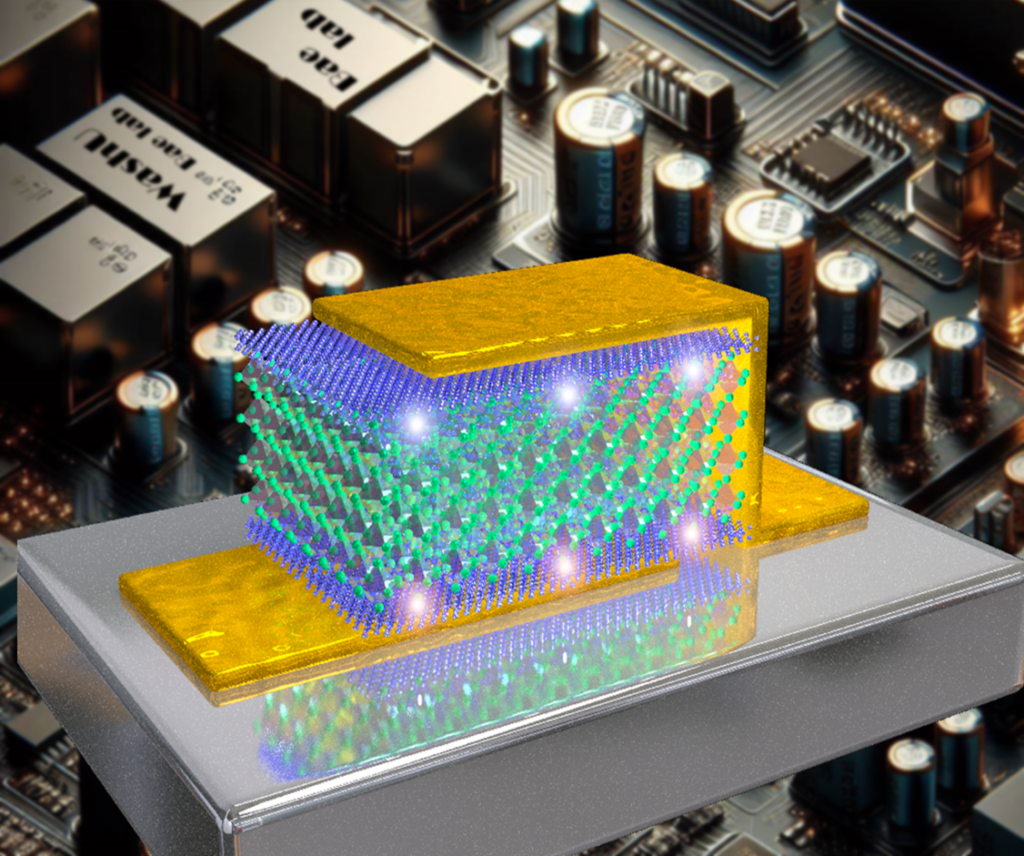
Sang-Hoon Bae, an assistant professor of mechanical engineering and materials science in the McKelvey School of Engineering at Washington University in St. Louis, has addressed this long-standing challenge in deploying ferroelectric materials for energy storage applications. In a study published April 18 in Science, Bae and his collaborators, including Rohan Mishra, associate professor of mechanical engineering and materials science, and Chuan Wang, associate professor of electrical and systems engineering, both at WashU, and Frances Ross, at Massachusetts Institute of Technology, introduced an approach to control the relaxation time — an internal material property that describes how long it takes for charge to dissipate or decay — of ferroelectric capacitors using 2D materials.
Working with Bae, doctoral student Justin S. Kim and postdoctoral researcher Sangmoon Han developed novel 2D/3D/2D heterostructures that can minimize energy loss while preserving the advantageous material properties of ferroelectric 3D materials. Their approach cleverly sandwiches 2D and 3D materials in atomically thin layers with carefully engineered chemical and nonchemical bonds between each layer. A very thin 3D core is inserted between two outer 2D layers to create a stack only about 30 nanometers thick — about one-tenth the size of an average virus particle.
Read more on the McKelvey School of Engineering website.


