Humans, animals, plants and bacteria all use heme. It is crucial for cytochrome proteins, which power the cell; cytochromes c use heme to carry electrons in respiration and photosynthesis. Robert Kranz, a professor of biology in Arts & Sciences at Washington University in St. Louis, has conducted research on heme synthesis, modification and transport for more than 30 years.

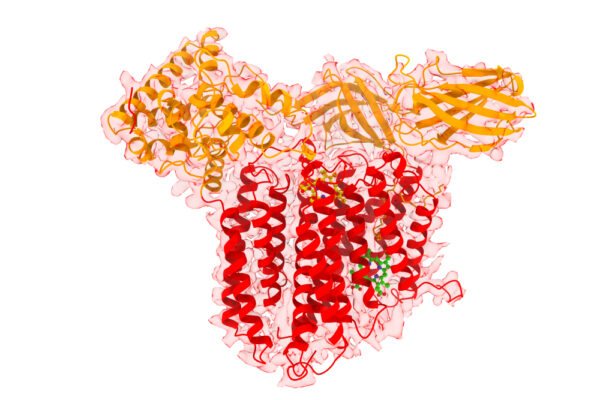
Kranz, staff scientist Deanna Mendez and Ethan Lowder, a 2021 alumnus, co-authored a paper published Oct. 28 in the journal Nature Communications that reports the structures and mechanisms of a type of ATP-dependent heme transporter, CcmABCD, at multiple states.
CcmABCD is present in many bacteria, archae and all plant mitochondria. Kranz discovered the genes for the CcmABCD complex in 1989 and his lab has published key genetic and biochemical papers on its function since then.
“This study brings to fruition a cryo-EM structural basis for this unique ATP-dependent heme release complex, required for cytochrome c maturation, called ccm,” Kranz said. “We also compared our previously published cryo-EM structures of a different heme transporter, CcsBA, and proposed some common mechanisms of heme trafficking in the cell.” The study is a collaboration with Jiapeng Zhu at Nanjing University and Kai Zhang at Yale and their groups.
Based on their structures — combined with functional studies — the researchers offer a hypothetical model of heme trafficking, heme transfer to CcmE and ATP-dependent release of holoCcmE from CcmABCD.